Abstract
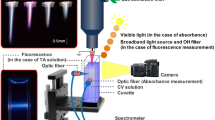
Radio frequency (RF) plasma in water was used for the degradation of methylene blue. The fraction of decomposition of methylene blue and the intensity of the spectral line from OH radical increased with RF power. RF plasma in water also produced hydrogen peroxide. The density of hydrogen peroxide increased with RF power and exposure time. When pure water (300 mL) is exposed to plasma at 310 W for 15 min, density of hydrogen peroxide reaches to 120 mg/L. Methylene blue after exposed to plasma degraded gradually for three weeks. This degradation may be due to chemical processes via hydrogen peroxide and tungsten. The comparison between the experimental and calculated spectral lines of OH radical (A–X) shows that the temperature of the radical is around 3,500 K. Electron density is evaluated to be ≃3.5 × 1020 m−3 from the stark broadening of the Hβ line.















Similar content being viewed by others
References
Clements JS, Sato M, Davis RH (1987) IEEE Trans Ind Appl 23:224
Sharma AK, Locke BR, Arce P, Finney WC (1993) Hazard Waste Hazard Mater 10:209
Joshi AA, Lock BR, Arce P, Finney WC (1995) J Hazard Matter 41:3
Grymonpre DR, Finney WC, Locke BR (1999) Chem Eng Sci 54:3095
Sahni M, Finnery WC, Locke BR (2005) J Adv Oxid Technol 8:105
Grymonpre DR, Sharma AK, Finney WC, Locke BR (2001) Chem Eng J 82:189
Inoue M, Okada F, Sakurai A, Sakakibara M (2006) Ultrasonics Sonochem 13:313
Pawłat J, Ihara S, Yamabe C, Pollo I (2005) Plasma Process Polym 2:218
Aoki H, Kitano K, Hamaguchi S (2007) Proceedings of 18th international symposium on Plasma Chemistry, Kyoto, Japan: 00339
Ishijima T, Hotta H, Sugai H, Sato M (2007) Appl Phys Lett 91:121501
Maehara T, Toyota H, Kuramoto M et al. (2006) Jpn J Appl Phys 45:8864
Nomura S, Toyota H (2003) Appl Phys Lett 83:4503
Nomura S, Toyota H, Mukasa S, Yamashita H, Maehara T, Kuramoto M (2006) Appl Phys Lett 88:211503
Takahashi Y, Toyota H, Nomura S, Mukasa S (2007) Proceedings of 18th international symposium on plasma chemistry, Kyoto, Japan: 00133
Takai O (2007) Proceedings of 18th international symposium on plasma chemistry, Kyoto, Japan: 00254
Levin DA, Laux CO, Kruger CH (1999) J Quant Spectrosc Radiat Transf 61:377
Mašláni A, Sember V (2004) Proceedings on fourth international workshop and school towards fusion energy-plasma physics, Diagnostics, Applications Kudowa Zdroj, Poland: 669
Luque J, Crosley DR, (1999) “LIFBASE” Database and Spectral Simulation Program (Version 1.5), SRI International Report Mp 99-009
Laux O, Spence TG, Kruger CH, Zare RN (2003) Plasma Source Sci Technol 12:125
Sember V, Gravelle DV, Boulos MI (2002) J Phys D: Appl Phys 35:1350
Stehlé C, Hutcheon R (1999) Astron Astrophys Suppl Ser 140:93
Tendero C, Tixier C, Tristant P, Desmaison J, Leprince P (2006) Spectrochimica Acta Part B 61:2
Griem HR (1964) Plasma spectroscopy. MacGrow-Hill, New York
Chase MW Jr, Davies CA, Downey JR Jr, Frurip DJ, McDonald RA, Syverud AN (1985) JANAF thermochemical tables, 3rd edn. American Chemical Society and the American institute of Physics for the National Bureau of Standards, New York
Seidell A, Linke WF (1965) Solubilities of inorganic and metal organic compounds, 4th edn. American Chemical Society, Washinton
Acknowledgements
The present work was partially supported by Grants-in Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan (No. 17654118) (T. Maehara) and (No. 17560636) (H. Toyota). The authors thank Mr. H. Okumura for technical support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maehara, T., Miyamoto, I., Kurokawa, K. et al. Degradation of Methylene Blue by RF Plasma in Water. Plasma Chem Plasma Process 28, 467–482 (2008). https://doi.org/10.1007/s11090-008-9142-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-008-9142-2