Abstract
Miniaturized atmospheric pressure glow discharges (APGDs) were generated in contact with small sized flowing liquid cathode systems. As anodes a solid pin electrode or a miniature flow Ar microjet were applied. Both discharge systems were operated in the open to air atmosphere. Hydrogen peroxide (H2O2) as well as ammonium (NH4 +), nitrate (NO3 −), and nitrite (NO2 −) ions were quantified in solutions treated by studied discharge systems. Additionally, an increase in the acidification of these solutions was noted in each case. Emission spectra of the near cathode zone of both systems were measured in order to elucidate mechanisms that lead to the formation of active species in gas and liquid phases of the discharge. Additionally, the concentration of active species in the liquid phase (H2O2, NH4 +, NO3 − and NO2 −) was monitored as a function of the solution uptake rate and the flow rate of Ar. The suitability of investigated discharge systems in the water treatment was tested on artificial wastewaters containing an organic dye (methyl red), hardly removable by classical methods non-ionic surfactants (light Triton x-45 and heavy Triton x-405) and very toxic Cr(VI) ions. Preliminary results presented here indicate that both investigated flow-through APGD systems may successfully be applied for the efficient and fast on-line continuous flow chemical degradation of toxic and hazardous organic and inorganic species in wastewater solutions.
Similar content being viewed by others
Introduction
Non-equilibrium atmospheric pressure discharges, sometimes termed as cold atmospheric plasmas (CAPs), atmospheric pressure plasmas (APPs) or non-thermal plasmas (NTPs), generated directly in liquids or in contact with liquids are frequently applied in cleaning, bio-decontamination, sterilization and environmental remediation processes of waste waters [1]. So far, different constructions and types of non-equilibrium atmospheric plasma sources, e.g., dielectric barrier discharges (DBDs), atmospheric pressure glow discharges (APGDs), atmospheric plasma jets (APJs), pulsed discharges, plasma needle or corona discharges, have successfully been employed in wastewater treatment processes [1–23].
Among them, APGDs generated in or in contact with liquids are established to be a useful source of various reactive oxygen (ROS) and nitrogen (RNS) species formed in gas and liquid phases of these discharges, i.e., hydrogen peroxide (H2O2), hydrogen peroxonitrate (O=NOOH), singlet oxygen (1O2) and ozone (O3) molecules, hydroxyl (OH), hydrogen (H), hydroperoxyl (O2H), nitrogen oxide (NO), and oxygen (O) radicals, superoxide (O2 −) ions as well as the UV irradiation [1, 6]. The production of ROS and RNS is the most important in the chemical degradation of hazardous chemicals in aqueous solutions as well as bio-decontamination of water [1, 7]. In particular, the OH radical and O2 − ions and H2O2 molecules seem to play a crucial role in the chemical decomposition of organic pollutants in wastewater solutions [1, 3]. Therefore, APGDs are quite suitable for removal processes of organic pollutants and bio-decontamination and sterilization purposes [3]. It was reported that this type of plasma sources was applied so far to the purification of water from organic dyes [8–11], phenol [12–14], or organic halides [15–17] and to the sterilization of water [1, 18] and its bio-decontamination [19–21]. The discoloration of wastewaters containing various dyes and the decomposition of other organic compounds by glow discharges (GDs) were rather frequently studied in various plasma reactors and electrode configurations, i.e., discharges in liquids termed as glow discharge electrolysis (GDE) [10, 16], discharges in contact with liquids termed as contact glow discharge electrolysis (CGDE) [6, 9, 12, 20–23] and discharges between two liquids separated by thin diaphragm holes termed as diaphragm glow discharge (DGD) [11, 17]. It was also possible to inactivate glutathione [20], cyanotoxins (microcystin-LR) [21] or cyanobacteria (microcystis aeruginosa) in fresh waters by CGDEs [22]. The removal of inorganic hazardous chemicals from waters by APGDs was faintly examined. Recently, Ke et al. [6] have been investigated reduction and removal processes of aqueous solutions of hexavalent Cr ions by APGD generated between a bulk liquid cathode and a solid anode in the Ar atmosphere. They found that the addition of a scavenger of OH radicals, e.g., ethanol, was very advantageous in the point of view of the reduction and the removal of Cr(VI) ions. In another work [23], the removal of Cr(VI) ions by CGDE sustained in the Ar atmosphere was studied in function of the solution pH. An enhanced reduction of Cr(VI) ions was observed for low solution pHs or when radical scavengers were added to solutions.
The main aim of the present work was to develop the new APGDs working in the flowing liquid mode to degradation of pollutants in waster water solution. To the best of our knowledge, this is the first time when such flow-through APGD systems, with small sized flowing liquid cathodes, were used for the decomposition of selected organic pollutants and the reduction of Cr(VI) ions. The suitability of both investigated discharge systems in wastewater treatment processes was also evaluated and studied in function of the solution flow rate and the flow rate of Ar used as the discharge gas in the system with the gas microjet. Additionally, the discharge–liquid interactions as well as the production yield of chemically active species in the discharge and the liquid phase were elucidated in order to clarify what elementary processes exist in two types of APGD sources.
Experimental
APGD Reactor Devices
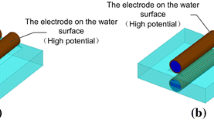
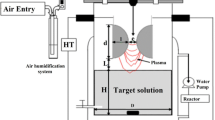
A schematic diagram of water treatment processes carried out with studied APGD systems is presented in Fig. 1. Both discharges were sustained in the open to air atmosphere. In both applied discharge devices small sized flowing liquid cathodes were used. Water solutions with and without pollutants were delivered to both systems through quartz tubes (i.d. 2 mm), onto which graphite tubes (i.d. 4 mm) were imposed in order to provide the electrical contact. Both APGDs were obtained by the direct-current (dc) discharge using as anodes a molybdenum rod (i.d. 2 mm, see Fig. 1a) or a miniature Ar flow passed through a steel nozzle (i.d. 0.5 mm, see Fig. 1b) and forming a gas microjet. The gap between electrodes was 5 mm in each case. The flow rate of Ar was controlled and adjusted with a Tylan-General flow meter system (a RO-28 flow meter with a F-2900 flow controller) and was maintained within the range of 60–300 sccm (cubic centimeter per minute at STP). A high voltage (HV) direct current (dc) glow discharge power supply (Dora, Wroclaw, Poland) with a maximum power output of 200 W was used in each case. Additionally, a ballast resistor (10 kΩ, 50 W, Tyco Electronics, USA) in the anode circuit was applied to stabilize the discharge current. The voltage supplied to electrodes was within the range of 1,100–1,500 V. This resulted in currents of 35–40 mA and stable glow type discharges operated in both studied systems at the atmospheric pressure. A peristaltic pump (LabCraft, France) was applied to deliver water solutions into discharge zones of both APGD systems. The flow rate of these solutions was maintained in the range from 0.6 to 3.2 mL min−1. Discharge systems were worked in a single-pass mode without the re-circulation of solutions. Water sample solutions (wastewaters and clean waters acidified with HCl), treated by both APGDs, were directly collected into 50-ml glass tubes and were analyzed using various methods of the analysis.
The Acquisition of Emission Spectra
Emission spectra of investigated APGD systems were recorded with a step of 0.02 nm and within the wavelength range from 200 to 900 nm. A TRIAX 320 (Horiba Jobin Yvon, France), 0.32 m scanning monochromator was used for that and it was equipped with a 1,200 grooves mm−1 diffraction grating blazed at 250 nm. As a radiation detector, a Hamamatsu R-928 photomultiplier biased at −700 V with a JY SpecAcq2 acquisition system were employed. The integration time of the photomultiplier was set at 0.1 s. The UV–Vis emission spectra of investigated APGDs were collected from their near liquid cathode zones, where plasma processes, responsible for the removal of toxic species, were the most intensive. The SpectraMax/32 computer program was applied to visualize and handle all measured data.
Reagents and Solutions
All reagents used were of analytical grade or better. Re-distilled water was used throughout. Stock solutions (5,000 mg L−1) of studied pollutants, i.e., methyl red (MR), non-ionic surfactants (Triton X-45 and Triton X-405) and hexavalent Cr(VI) ions (as potassium dichromate, K2Cr2O7), were prepared by dissolving required amounts of respective reagents in water. MR (2-(N,N-dimethyl-4-aminophenyl)azobenzenecarboxylic acid, C15H15N3O2) and K2Cr2O7 were purchased from POCh (Poland). Solid Triton x-45 (C8H17C6H4O(C2H4O)4.5H) and a 70 % (m/v) water solution of Triton x-405 (C8H17C6H4O(C2H4O)35H) were provided by Sigma-Aldrich (Germany). Working water solutions of pollutants were prepared from their stock solutions by appropriate dilutions with water. The conductivity of all liquid samples was adjusted with a 37 % (m/m) HCl solution (POCh, Gliwice, Poland). The final concentration of HCl in these solutions was 0.10 mol L−1. The initial concentration of pollutants, i.e., MR, Triton X-45, Triton X-405 and Cr(VI) ions were 30, 150, 1,600 and 100 mg L−1, respectively, and corresponded to average concentrations of these compounds in different waste waters. High purity Ar (99.998 %, Messer, Poland) was applied in case of the APGD system with the Ar microjet.
The analysis of the hydrogen peroxide as well as nitrate, nitrite and ammonium ions concentrations and overall acidity in water solutions treated by discharges.
The composition of water solutions treated with both discharges and overflowed delivery tubes and collected in waste reservoirs of discharge cells was analyzed. The concentration of H2O2 was measured by means of the spectrophotometric method with ammonium metavanadate (NH4VO3) in the acidic medium [10]. Accordingly, in the reaction of H2O2 with VO3 − ions, the red-orange peroxovanadium cation (VO2 3+) was formed with the maximum absorption at the wavelength of 450 nm. The total concentration of H3O+ ions (the overall acidity) in water solutions were determined by the acid–base titration using a NaOH solution (0.1000 mol L−1) as a standard-titer and phenolphthalein (0.1 %) as an indicator, both purchased from POCh, Gliwice, Poland. The concentration of nitrite (NO2 −) ions was determined by the rivanol method [24] using a Metertek (Poland) spectrophotometer, model SP-850. The absorbance of a cherry-red complex resulted from the reaction of NO2 − ions with rivanol (2-ethoxy-6,9-diaminoacridinium lactate) in the medium of 0.1 mol L−1 HCl was measured at the wavelength of 590 nm. An Orion (USA) ion selective electrode was used to measure the concentration of NO3 − ions. The Nessler’s method was applied for the spectrophotometric determination of the concentration of ammonium (NH4 +) ions [25]. In each case, appropriate external calibration curves were prepared and used for the quantification of studied species. The accuracy and the precision of applied methods were verified by the analysis of check sample solutions, containing known amounts of all species (n = 3). It was found that the accuracy, defined as the relative error (RE), was in the range −2 % to +3 %, while the precision, defined as the relative standard deviation (RSD), was within 1–5 %.
The Examination of the Chemical Degradation of Organic and Non-organic Species
The yield of the discoloration of MR and the decomposition of Triton X-45 and X-405 non-ionic surfactants was verified by means of UV–Vis spectrophotometry measurements. A Thermo Scientific GENESYS 10 scanning UV/Vis spectrophotometer and UV quartz cuvettes were used. The absorbance of a MR solution was measured at λ = 515 nm that corresponds to the maximum of its absorption band. The absorption spectra of Triton X-45 and X-405 solutions were collected in the range 190–310 nm (step 1 nm). Two characteristic absorption peaks were visible at ~225 and ~275 nm [26]. The concentration of Cr(VI) ions in water samples was measured spectrophotometrically, applying the reaction of Cr(VI) ions with 1,5-diphenylcarbazide in the acetone medium [25]. The absorbance of the product of the reaction of 1,5-diphenylcarbazide with Cr(VI) ions was measured at λ = 540 nm. The limit od detection (LOD) of this method was estimated to be below 4 μg L−1 of Cr(VI).
Results and Discussion
The Formation of Active Species in the Gas Phase of APGD System
In order to identify active components of both discharges, i.e., atoms, ions or molecules, emission spectra of investigated APGD systems were recorded within the spectral range of 200–900 nm, focusing the radiation emitted from the region near the liquid cathode (the near-cathode region). Emission spectra of both electrode systems, i.e., the solid metallic anode—the flowing liquid cathode and the Ar microjet—the flowing liquid cathode, are shown in Fig. 2. Both discharge systems were fed with prepared solutions of studied pollutants at the same flow rate of 1.2 mL min−1. As can be seen, the UV spectra within 200–400 nm are dominated by the emission of NO bands belonging to the γ-system (the transition A2Σ+–X2Π) and bands of the OH radical belonging to the A2Σ–X2Π system. Strong bands of N2, belonging to the second positive system (C3Πu–B3Πg), and bands of NH (A3Π–X3Σ−), with band heads at 336.0 and 337 nm, were also excited in both systems. Atomic lines ascribed to H lines of the Balmer series (Hα at 656.2 nm, Hβ at 486.2 nm, Hγ at 431.4 nm) and O atomic lines (O I at 777.2, 777.4 and 844.6 nm) were detected in the wavelength range of 400–900 nm. In this spectral range, bands of N2, belonging to strong C3Πu–B3Πg and weak B3Πg–A3Σ +u systems, were also identified. Atomic lines of Ar with excitation energies from 13 to 15 eV, identified in the spectral range from 680 to 900 nm of the APGD system operated with the Ar microjet, were also excited.
It should be noted that, the intensity of NH, NO and N2 emission bands identified in spectra of the APGD system generated between the Ar microjet and the flowing liquid cathode were considerably higher than those recorded for the APGD system generated between the solid metallic anode and the flowing liquid cathode. Apparently, in the first discharge system, metastable states of Ar (Arm), with the excitation energy of ~11 eV, could participate in the production of N2, NH and NO excited states [27]. The intensity of the OH emission was higher in the pin electrode system, but the emission of H and O atomic lines was comparable for both studied systems. Due to the nature of APGD generated in contact with the liquid cathode, the main source of OH radicals was likely to be H2O+/H3O+ ions and/or the water vapor [2, 28]. In addition, N2 molecules possibly diffused to the core of both discharges from the surrounding air atmosphere [2]. It was presumed that NH and NO molecules were produced in discharge zones as a result of the reaction of active N2 with H and O radicals, respectively [2, 27]. Neither carbon diatomic molecules, i.e., CH, C2, CO, CO+, CN, nor atomic lines of C were identified in emission spectra of both APGD systems generated in contact with solutions containing organic compounds, i.e., non-ionic surfactants and the organic dye. It may indicate that the transport of organic species to discharge zones was hindered. On the other hand, the quenching of the emission of carbon diatomic molecules by water vapor molecules could also happen.
Quite different results were obtained when both APGD systems were fed with water solutions containing Cr(VI) ions. In this case, strong atomic emission lines of Cr, having excitation energies within the range of 2.9–3.4 eV, were identified in emission spectra. The most prominent emission lines of Cr I were observed at 357.9, 359.3, 360.5, 425.4, 427.5 and 429.0 nm. Probably, the main mechanism responsible for the transport of Cr atoms from the liquid to the discharge phase of both APGD systems was the sputtering of water solutions. This processes likely occurred due to the bombardment of the surface of these solutions by heavy ions existed in the discharge, e.g., H2O+ [28]. Due to a high temperature of the discharge (~2,000–4,000 K) [2, 27, 28], the transport of Cr atoms to the discharge phase through the evaporation of water could also be taken into consideration.
The Formation of Species in the Liquid Phase of APGD System
Hydrogen peroxide (H2O2) as well as nitrate (NO3 −) ions and traces of nitrite (NO2 −) and ammonium (NH4 +) ions were identified in water solutions, with or without studied pollutants, treated by APGDs generated in both electrode systems. Additionally, the acidification of these solutions was noted. It should be stressed that H2O2 molecules and NO3 −, NO2 − and NH4 + ions were not detected in water solutions untreated by both APGD systems. Changes in the concentration of H2O2 and the overall acidity for different flow rates of 0.1 mol L−1 HCl solutions are given in Fig. 3 for water without studied pollutants. Generally, the highest concentration of H2O2, i.e., 38 and 19 mg L−1, respectively, for APGD systems with the solid metallic anode and the Ar microjet, was noted at relatively low solution flow rates (0.9–1.3 mL min−1). This was probably due to a longer contact time of the surface of solutions with phases of both discharges. Indeed, the growth of the solution flow rate to 3.2 mL min−1 was found to result in a decrease in the concentration of H2O2 by about 30 %, i.e., to 25 and 13 mg L−1, for discharges with the solid metallic anode and the Ar microjet, respectively. This was probably associated with a shorter exposure of solutions to the discharge treatment. The H2O2 generation energy yield and the generation rate were estimated to be 0.05–0.13 g kWh−1 and 0.002–0.005 g h−1, respectively, for the solid anode-liquid cathode and 0.03–0.07 g kWh−1 and 0.002–0.003 g h−1, respectively, for the microjet anode-liquid cathode system. These values were comparable with another plasma system generated in contact with liquid [29]. Contrary, the generation energy yield as well as the generation rate increased with the growth of the solution flow rate. It may probably be connected with an enhanced evaporation of water at a longer exposure of a solution to the discharge. Additionally, a 40 % increase in the overall acidity of solutions treated by both discharges was observed in each case. Concentrations of NO3 −, NO2 − and NH4 + ions in water solutions treated by both APGD systems under different experimental conditions are collected in Fig. 4. It was established that concentrations of NO3 −, NO2 − and NH4 + ions decreased with an increasing flow rate of solutions treated by both discharge systems.
It should be noted that concentrations of NO3 − and NO2 − ions in water solutions treated by the discharge with the Ar microjet was higher than those determined in water solutions treated by the discharge with the solid metallic anode. The concentration of NH4 + ions measured in water solutions treated by both APGD systems was comparable. The growth of the flow rate of Ar from 60 to 300 sccm in the case of the Ar microjet anode caused an increase in concentrations of NO3 − and NO2 − ions and an enhancement of the overall acidity of solutions. At the same time, the H2O2 concentration was decreased. This may indicate that high excited or metastable Ar species, produced in the APGD system with the miniature Ar flow microjet, could participate in the production of nitrogen like species (NO, NO2) in the gas phase of the discharge. It can be presumed that the main source of NO2 − and NO3 − ions were nitric oxide (NO) and nitric dioxide (NO2), respectively, which reacted with OH radicals in discharge phases according to the following reactions: NO + OH = HNO2; NO2 + OH = HNO3. However, at low pH, NO2 − ions could be oxidized to NO3 − ions (3HNO2 = NO3 − + 2NO + H3O+) or decomposed (2HNO2 = NO + NO2 + H2O) [7] and thus concentrations of NO2 − ions determined were relative low, i.e., ~0.5 mg L−1. Under acidic conditions hydrogen peroxonitrate (ONOOH) could also be produced from NO2 − ions and H2O2 according to the following reaction: NO2 − + H2O2 + H3O+ = ONOOH + 2H2O [7]. Nitric acid (HNO3) was mainly responsible for the acidification of water solutions treated with the APGD system with the Ar microjet [30]. On the other hand, the flow rate of Ar was found to have a little influence on the production of NH4 + ions. Heterogeneous reactions, taking place on the reactor wall or the steel nozzle, were presumably responsible for the production of NH3 molecules. In this process, NH and H radicals (identified in emission spectra of both discharges) could also play an important role [27]: NHx + H = NHx+1, where x = 0–2. Resulting NH3 molecules could be the source of NH4 + ions in the liquid phase of both discharges.
The following species: H2O2, NO2 − and NO3 − have recently been identified by Shimizu et al. [5] in a water solution treated by an APGD system sustained in the air atmosphere between a solid anode and a bulky liquid cathode. Molecules of H2O2 were likely to be produced in oxidation processes of H2O molecules or species originated from them, e.g., vibrationally excited water molecules (H2O*), OH radicals, or H2O+ ions, in the following reactions: OH + OH = H2O2; OH + H2O* = H2O2 + H; 2H2O+=H2O2 + 2H+; 2H2O* = H2O2 + H2 [29].
The Application of Both APGDs System in Wastewater Treatment Processes
The application of both discharges for the wastewater purification was tested in solutions containing three different pollutants, i.e., the organic dye (methyl red), non-ionic surfactants (Triton X-45 and Triton X-405) and very toxic inorganic Cr(VI) ions. The degradation efficiency of pollutants (η) was calculated using the equation: η = 100 % × (Co−Ct)/Co, where Co is the initial concentration of the pollutant in the untreated solution, Ct is the final concentration of the pollutant in the solution treated by the discharge. In the case of non-ionic surfactants, the degradation efficiency was only roughly estimated considering UV absorption spectra of solutions containing studied surfactants. Perkowski et al. [31, 32] reported a quite similar approach.
APGD Treatment of Solutions Containing Methyl Red Dye and Non-ionic Surfactants
As can be seen from Fig. 5, the increase in the solution flow rate up to 3.3 mL min−1 was established to produce a falling of the degradation efficiency of MR from 99 to 72 % and from 98 to 88 %, respectively, for the discharge system with the solid anode-the flowing liquid cathode and the Ar microjet-the flowing liquid cathode. Moreover, it was found that the flow rate of Ar had a negligible influence on the degradation efficiency of MR (~5 %). The first step of the oxidation of MR occurs by the adjunction of the OH radical to the aromatic C atom, breaking the azo bond (–N=N–) [33, 34] since it is sensitive to the oxidation by OH radicals [35]. The resulting OH adduct tends to break down and derivatives of phenyldiazene and phenoxy radicals are produced. Next, decomposition products of MR, i.e., derivatives of phenyldiazene and phenoxy radicals, may be transformed into other low molecular weight organic compounds [33, 34].
UV absorption spectra of untreated and APGD treated solutions of non-ionic surfactants, i.e., Triton X-45 (n = 4.5) and Triton X-405 (n = 35), are shown in Fig. 6a, b, respectively. The first broad absorption band (within 190–240 nm) in UV spectra of lighter and higher non-ionic surfactants, with peaks at around 200 nm and 225 nm, may be assigned to an ethylene oxide chain (–CH2–CH2–O–) and aromatic rings of benzene. The next broad peak at 275 nm may be assigned to benzene derivatives [31]. As can been seen from Fig. 6a, for the lighter non-ionic surfactant Triton X-45, the discharge treatment of its solutions results in the disappearance of peaks at 200, 225 and 275 nm in absorption spectra. Absorption spectra of Triton X-45 solutions treated with both APGD systems are comparable to this acquired for water (measured as the control sample). This points out that the degradation efficiency of Triton X-45 in solutions treated by both APGD sources is almost 100 %. Taken into account only the absorption at 225 nm [26], the rough degradation efficiency of Triton X-45 was estimated to be 100 % and 92 %, respectively, for solid anode-liquid cathode and Ar microjet anode-liquid cathode systems. Quite different results were obtained for the heavier non-ionic surfactant Triton X-405, which contains a very long polyethylene oxide chain (the mean value of the chain n = 35). In absorption spectra of solutions of this surfactant treated with both APGD systems only peaks at 200 and 225 nm were found to extinguish. Unfortunately, the absorption of the band at 275 nm identified in untreated and discharge treated solutions of Triton X-405 was comparable. Moreover, in UV spectra of Triton X-405 solutions treated by both discharges, the band at 235 nm was also observed. It could be presumed that ROS (e.g., OH radicals) and/or electrons produced in the liquid–gas interface of both discharge systems may cause the shortening of ethylene oxide chain species and the opening of benzene rings in the Triton X-45 surfactant [31, 32]. In the case of heavy Triton X-405 only the shortening of the ethylene oxide chain may happen.
APGD Treatment of Solutions Containing Hexavalent Cr Ions
The dependence of the degradation efficiency of Cr(VI) ions from the solution flow rate is presented in Fig. 7. As can be seen, in the range of the solution flow rate from 0.9 to 1.9 mL min−1, a sudden fall of the degradation efficiency of Cr(VI) from 93 to 50 % was noted for both discharges. A further increase of the solution flow rate up to 3.2 mL min−1 did not cause any additional changes of the degradation efficiency, which remains at the same level (~50 %). Contrary, the growth of the Ar flow rate from 60 to 300 sccm in the APGD system with the Ar microjet was found to improve the degradation of Cr(VI) ions by about 20 % at the solution flow rate of 1.2 mL min−1. The energy efficiency (G) for the reduction of Cr(VI) ions was estimated to be (0.5–0.9) × 10−4 mg J−1 for both APGD systems. It should be noted that the highest energy efficiency was noted at the solution flow rate of 3.2 mL min−1. Slightly higher and higher values of the energy efficiency were calculated by Wang and Jiang [23] and Ke et al. [6], respectively, for glow discharge systems generated in contact with bulky liquid cathodes.
According to Wang and Jiang [23] and Ke et al. [6], H radicals and hydrated electrons (e) may participate in the reduction of Cr(VI) ions in the liquid phase, e.g., Cr2O7 2−+6H + 8H3O+ = 2Cr3+ + 15H2O or Cr2O7 2− + 6e + 14H3O+ = 2Cr3+ + 21H2O. Moreover, H2O2 in the acid medium can also contribute to the reduction of hexavalent Cr ions to their trivalent forms, i.e., Cr(III) ions, in the following reaction: 3H2O2 + Cr2O7 2− + 8H+ = 3O2 + 2Cr3+ + 7H2O. On the other hand, highly oxidative species (e.g., OH radicals) can re-oxidize lower states of Cr to higher states and thus, the addition of radical scavengers to solutions is very advantageous for the efficient reduction of Cr(VI) ions [6, 23]. Such a radical scavenger chosen in the present work was ethyl alcohol (1 % v/v). It was found that the removal efficiency of Cr(VI) ions from solutions containing ethyl alcohol and treated by both APGD systems was 100 % at all solution flow rates. It should also be noticed that the addition of ethyl alcohol to solutions containing up to 500 mg L−1 hexavalent Cr ions and treated by both APGD sources caused the reduction of Cr(VI) ions with the degradation efficiency of 100 % at the solution flow rate within 0.9–2 mL min−1.
Conclusions
Two kinds of APGD systems, i.e., the solid metallic anode—the small sized flowing liquid cathode and the Ar microjet anode—the small sized flowing liquid cathode, were studied and compared in reference to the degradation of dangerous chemical species in waste waters as well as production yield of active species in discharge and liquid phases. The degradation efficiency of pollutants studied here (methyl red dye, Triton X-45 and X-405 non-ionic surfactant and hexavalent Cr ions) was found to be within 50–100 % and strongly depended on the solution flow rate. The following species: OH, NH, NO, N2, H and O were excited in both discharges. Hydrogen peroxide in addition to nitrite, nitrate and ammonium ions were identified in water sample solutions treated by both discharges as a result of different chemical reactions occurred due to the interaction of these discharges with water solutions. Additionally, a growth in the overall acidity of the liquid phase was noted for both discharge systems. Although, overflowing liquid cathodes were applied in both discharge systems and thus, the contact of discharges with treated solutions was relatively short, it seems that both APGD systems are very promising in the efficient on-line flow-through degradation of pollutants in wastewaters.
References
Yang Y, Cho YI, Friedman A (2012) Plasma discharge in liquid: water treatment and applications. CRC Press, Boca Raton
Jamroz P, Zyrnicki W (2011) Plasma Chem Plasma Process 31:681–696
Wang X, Zhou M, Jin X (2012) Electrochim Acta 83:501–512
Magureanu M, Bradu C, Piroi D, Mandache NB, Parvulescu V (2013) Plasma Chem Plasma Process 33:51–64
Shimizu T, Iwafuchi Y, Morfill GE, Sato T (2011) New J Phys 13:053025
Ke Z, Huang Q, Zhang H, Yu Z (2011) Environ Sci Technol 45:7841–7847
Machala Z, Tarabova B, Hensel K, Spetlikova E, Sikurova L, Lukes P (2013) Plasma Process Polym 10:649–659
de B. Benetoli LO, Cadorin BM, da S. Postiglione C, de Souza IG, Debacher NA (2011) J Braz Chem Soc 22:1669–1678
Jin X, Zhang H, Wang X, Zhou M (2012) Electrochim Acta 59:474–478
Nemcová L, Nikiforov A, Leys C, Krcma F (2011) IEEE Trans Plasma Sci 39:865–870
Jin X, Wang X, Zhang H, Ren H (2013) Electrochim Acta 87:336–340
Liu Y, Wang D, Sun B, Zhu X (2010) J Hazard Mater 181:1010–1015
Wang L, Jiang X, Liu Y (2008) J Hazard Mater 154:1106–1114
Gao J, Liu Y, Yang W, Pu L, Yu J, Lu Q (2003) Plasma Sources Sci Technol 12:533–538
Yang H, Tezuka M (2011) J Environ Sci 23:1044–1049
Lu Q, Yu J, Gao J (2006) J Hazard Mater 136:526–531
Wang L (2009) Plasma Chem Plasma Process 29:241–250
Ikawa S, Kitano K, Hamaguchi S (2010) Plasma Process Polym 7:33–42
Machala Z, Janda M, Hensel K, Jedlovsky I, Lestinska L, Foltin V, Martisovits V, Morova M (2007) J Molec Spectrosc 243:194–201
Ke Z, Yu Z, Huang Q (2013) Plasma Process Polym 10:181–188
Zhang H, Huang Q, Ke Z, Yang L, Wang X, Yu Z (2012) Water Res 46:6554–6562
Jin XL, Xia Q, Wang XY, Yue JJ, Wei DB (2011) Plasma Chem Plasma Process 31:697–705
Wang L, Jiang X (2008) Environ Sci Technol 42:8492–8497
Brabcová M, Rychlovský P, Němcová I (2003) Anal Lett 36:2303–2316
Clesceri LS, Greenberg AE, Eaton AD (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, Washington
Arslan-Alaton I, Erdinc E (2006) Water Res 40:3409–3418
Jamroz P, Zyrnicki W, Pohl P (2012) Spectrochim Acta Part B 73:26–34
Jamroz P, Greda K, Pohl P (2012) TrAC-Trends Anal Chem 41:105–121
Locke BR, Shih KY (2011) Plasma Sources Sci Technol 20:034006
Brisset JL, Benstaali B, Moussa D, Fanmoe J, Njoyim-Tamungang E (2011) Plasma Sources Sci Technol 20:034021
Perkowski J, Mayer J, Kos L (2005) Fibres Text East Eur 50:81–85
Perkowski J, Bulska A, Jóźwiak WK (2005) Environ Prot Eng 31:61–75
Spadaro JT, Isabelle L, Renganathan V (1994) Environ Sci Technol 28:1389–1393
Magureanu M, Mandache NB, Parvulescu VI (2007) Plasma Chem Plasma Process 27:589–598
Ruma, Lukes P, Aoki N, Spetlikova E, Hosseini SHR, Sakugawa T, Akiyama H (2013) J Phys D Appl Phys 46:125202
Acknowledgments
The work was financed by a statutory activity subsidy from the Polish Ministry of Science and Higher Education for the Faculty of Chemistry of Wrocław University of Technology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Jamróz, P., Gręda, K., Pohl, P. et al. Atmospheric Pressure Glow Discharges Generated in Contact with Flowing Liquid Cathode: Production of Active Species and Application in Wastewater Purification Processes. Plasma Chem Plasma Process 34, 25–37 (2014). https://doi.org/10.1007/s11090-013-9503-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-013-9503-3