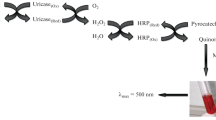
The conditions for spectrophotometric determination of mesalazine in urine using 7-chloro-4,6-dinitrobenzofuroxane as an analytical reagent have been established. The optimum analytical results are achieved at a wavelength of 500 nm for a solution with pH 6 – 8. The detection limit for mesalazine is 0.31 μg/mL. The possibility of using the proposed method for assessing mesalazine acetylation in metabolic processes by individual human phenotypes has been shown.







Similar content being viewed by others
References
V. G. Kukes, S. V Grachev, D. A. Sychev, and G. V. Ramenskaya, Drug Metabolism. Scientific Bases for Personalized Medicine [in Russian], GEOTAR-Media, Moscow (2008).
S. Yu. Garmonov, M. I. Evgen’ev, and I. E. Zykova, in: Problems in Analytical Chemistry, Vol. 11, Chemical Analysis in Medical Diagnostics [in Russian], Nauka, Moscow (2010), pp. 21 – 65.
P. G. Javier, G. Fernando, M. Jose, and M. P. Jose, Dig. Dis. Sci., 47(3), 471 – 488 (2002).
W. J. Sandborn and S. B. Hanauer, Aliment. Pharmacol. Ther., 17, 29 – 42 (2003).
H. Luck, M. Kinzig, A. Jetter, et al., Eur. J. Clin. Pharmacol., 65, 47 – 54 (2009).
H. J. Pieniaszek, Jr., and T. R. Bates, Res. Commun. Chem. Pathol. Pharmacol., 12(3), 571 – 581 (1975).
J. Tjornelund and S. H. Hansen, J. Chromatogr. B: Biomed. Sci. Appl., 570(1), 109 – 117 (1991).
G. Palumbo, G. Carlucci, P. Mazzeo, et al., J. Pharm. Biomed. Anal., 14(1 – 2), 175 – 180 (1995).
F. N. Hussain, R. A. Ajjan, M. Moustafa, et al., J. Chromatogr. B: Biomed. Sci. Appl., 716(1 – 2), 257 – 266 (1998).
B. Beata, N. Jolanta, and B. Jerzy, J. Pharm. Biomed. Anal., 22(2), 341 – 347 (2000).
P. Giancarlo, B. Simona, P. Luisa, et al., Biomed. Chromatogr., 19, 350 – 354 (2005).
M. Nobilis, Z. Vybiralova, K. Sladkova, et al., J. Chromatogr. A, 1119, 299 – 308 (2006).
P. Elisabetta, L. Marcello, S. Patrizia, et al., J. Chromatogr. B: Biomed. Sci. Appl., 872(1 – 2), 99 – 106 (2008).
A. S. Bailey and J. R. Case, Tetrahedron, 3, 113 – 131 (1958).
S. Yu. Garmonov, N. S. Shitova, A. V. Yakovleva, and R. A. Yusupov, Khim.-farm. Zh., 42(8), 49 – 53 (2008).
M. I. Evgen’ev, S. Yu. Garmonov, L. Sh. Shakirova, and A. S. Brysaev, Khim.-farm. Zh., 33(5), 50 – 53 (1999).
Author information
Authors and Affiliations
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 45, No. 12, pp. 48 – 51, December, 2011.
Rights and permissions
About this article
Cite this article
Garmonov, S.Y., Nguyen, Z.C., Mingazetdinov, I.F. et al. Spectrophotometric determination of mesalazine in urine for assessing the acetylation phenotype in vivo in humans. Pharm Chem J 45, 757–760 (2012). https://doi.org/10.1007/s11094-012-0719-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-012-0719-y