No Heading
Purpose.
Polystyrene sulfonate (PSS) is a novel noncytotoxic antimicrobial contraceptive agent. A gel formulation of PSS was found safe for vaginal administration in phase I clinical trials. The purpose of the current study was to develop and evaluate novel bioadhesive vaginal film formulations of PSS.
Methods.
PSS films were prepared by solvent evaporation and optimized for various physical, mechanical, and aesthetic properties. Further, films were evaluated for various biological activities and safety.
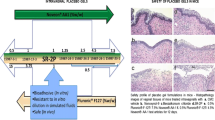
Results.
Vaginal films containing 300 mg PSS per unit have been developed, using generally regarded as safe (GRAS) listed excipients. The films are colorless, transparent, thin, soft, and tough, dissolve rapidly in physiologic fluid to form a smooth, viscous and bioadhesive solution that could be retained in the vagina for prolonged intervals. Sperm function inhibition (hyaluronidase and cervical mucus penetration) and antimicrobial activities against human immunodeficiency virus (HIV) and herpes simplex virus (HSV) by PSS films were found comparable to PSS. Also, films did not inhibit normal vaginal microflora (Lactobacillus) and were noncytotoxic as indicated by negligible sperm immobilization and cytotoxicity to host cell assays.
Conclusions.
Rapidly dissolving bioadhesive vaginal film formulation of PSS with desired physical, mechanical, aesthetic, and biological properties is a suitable candidate vaginal microbicide for prevention of sexually transmitted disease (STDs) and is ready for toxicological and clinical evaluation.
Similar content being viewed by others
Abbreviations
- AIDS:
-
acquired immunodeficiency syndrome
- ANOVA:
-
analysis of variance
- AUC:
-
area under curve
- DSC:
-
differential scanning calorimetry
- GPC:
-
gel permeation chromatography
- GRAS:
-
generally regarded as safe
- HEC:
-
hydroxyethyl cellulose
- HIV:
-
human immunodeficiency virus
- HPMC:
-
hydroxypropylmethyl cellulose
- HPV:
-
human papilloma virus
- HSV:
-
herpes simplex virus
- IC50:
-
inhibitory concentration at 50% level
- IC99.9:
-
inhibitory concentration at 99.9% level
- MW:
-
molecular weight
- N-9:
-
nonoxynol-9
- PEG:
-
polyethylene glycol
- PFU:
-
plaque forming unit
- PG:
-
propylene glycol
- PSS:
-
polystyrene sulfonate
- PVA:
-
polyvinyl alcohol
- RH:
-
relative humidity
- SD:
-
standard deviation
- SEM:
-
standard error mean
- SNK test:
-
Student-Newman-Keuls test
- STDs:
-
sexually transmitted diseases
- SVFM:
-
modified simulated vaginal fluid
- TGA:
-
thermogravimetric analysis
References
1. AIDS Epidemic update: December 2002 and December 2003. Available at http://www.unaids.org/Unaids/EN/Resources/Publications/corporate+publications/aids+epidemic+update+-+december+2003.asp (accessed 01/09/2004).
2. HIV Infection in Women. Available at http://www.niaid.nih.gov/factsheets/womenhiv.htm (accessed 01/09/2004).
3. P. F. Harrison. Microbicides: forging scientific and political alliances. AIDS Patient Care STDS 14:1–7 (2000).
4. J. R. Stephenson. Widely used spermicide may increase, not decrease, risk of HIV transmission. Med. News Persp. 284:949 (2000).
5. L. J. D. Zaneveld, R. A. Anderson, X. H. Diao, P. R. Young, D. P. Waller, S. Garg, and C. J. Chany. Method for preventing sexually transmitted diseases. US Patent No. 6239182, May 29, 2001.
6. R. A. Anderson, K. Feathergill, X. Diao, M. Cooper, R. Kirkpatrick, P. Spear, D. P. Waller, C. Chany, G. F. Doncel, B. C. Herold, and L. J. D. Zaneveld. Evaluation of poly(styrene-4-sulphonate) as a preventive agent for conception and sexually transmitted diseases. J. Androl. 21:862–875 (2000).
7. B. C. Herold, N. Bourne, D. Marcellino, R. Kirkpatrick, L. J. D. Zaneveld, D. P. Waller, R. A. Anderson, C. J. Chany, B. J. Barham, L. R. Stanberry, and M. D. Cooper. Poly(sodium 4-styrene sulphonate): an effective candidate topical antimicrobial for the prevention of sexually transmitted diseases. J. Infect. Dis. 181:770–773 (2000).
8. J. A. Simoes, D. M. Citron, A. Aroutcheva, R. A. Anderson, C. J. Chany, D. P. Waller, S. Faro, and L. J. D. Zaneveld. Two novel vaginal microbicides (polystyrene sulfonate and cellulose sulfate) inhibit Gardnerella vaginalis and anaerobes commonly associated with bacterial vaginosis. Antimicrob. Agents Chemother. 46:2692–2695 (2002).
9. N. D. Christensen, C. A. Reed, T. D. Culp, P. L. Hermonat, M. K. Howett, R. A. Anderson, and L. J. D. Zaneveld. Papillomavirus microbicidal activities of high molecular weight cellulose sulfate, dextran sulfate and polystyrene sulfonate. Antimicrob. Agents Chemother. 45:3427–3432 (2001).
10. L. J. D. Zaneveld, D. P. Waller, R. A. Anderson, C. Chany, W. F. Rencher, K. Feathergill, X. H. Diao, G. F. Doncel, B. Herold, and M. Cooper. Efficacy and safety of a new vaginal contraceptive formulation containing high molecular weight poly(sodium 4-styrenesulfonate). Biol. Reprod. 66:886–894 (2002).
11. Alliance for Microbicides Development Database. Available at https://secure.microbicide.org/DesktopDefault.asp?tabindex= 4&tabid=8 (accessed 01/09/2004).
12. J. Robinson and W. J. Bologna. Vaginal and reproductive system treatments using a bioadhesive polymer. J. Control. Rel. 28:87–94 (1994).
13. K. Vermani and S. Garg. The scope and potential of vaginal drug delivery. Pharm. Sci. Technol. Today 3:359–364 (2000).
14. K. Vermani, R. Kandarapu, G. Kohli, L. J. D. Zaneveld, and S. Garg. In vitro method to quantitate ‘retention’ of vaginal formulations, 5th International Symposium on Pharmaceutical Sciences and Technology, Mumbai, India, Feb 1–3 (2003).
15. D. F. Katz, M. H. Henderson, D. H. Owen, A. M. Plenys, and D. K. Walmer. What is needed to advance vaginal formulation technology? In W.F. Rencher (ed.), Vaginal Microbicide: Formulation Workshop, Lippincott Raven, Philadelphia, 1998, pp. 90–99.
16. C. Coggins, C. J. Elias, R. Atisook, M. T. Bassett, V. Ettiegnene-Traore, P. D. Ghys, L. Jenkins-Woelk, E. Thongkrajai, and N. L. VanDevanter. Women’s preferences regarding the formulation of over-the-counter vaginal spermicides. AIDS 12:1389–1391 (1998).
17. C. K. Mauck, S. Allen, J. M. Baker, S. P. Barr, T. J. Abercrombie, and D. F. Archer. An evaluation of the amount of nonoxynol-9 remaining in the vagina up to 4 h after insertion of vaginal contraceptive film (VCF®) containing 70 mg nonoxynol-9. Contraception 56:103–110 (1997).
18. K. Vermani, K. R. Tambwekar, C. Chany, L. J. D. Zaneveld, and S. Garg. Development and validation of GPC method for polystyrene sulfonate (PSS), a novel contraceptive antimicrobial agent, 53rd Indian Pharmaceutical Congress, New Delhi, Dec 21–23 (2001).
19. D. H. Owen and D. F. Katz. A vaginal fluid simulant. Contraception 59:91–95 (1999).
20. K. Vermani, S. Garg, and L. J. D. Zaneveld. Assemblies for in vitro measurement of bioadhesive strength and retention characteristics in simulated vaginal environment. Drug Dev. Ind. Pharm. 28:1133–1146 (2002).
21. S. Garg, K. Vermani, G. Kohli, R. Kandarapu, K. R. Tambwekar, D. P. Waller, and L. J. D. Zaneveld. Survey of vaginal formulations available in Indian market; physico-chemical characterization of selected products. Int. J. Pharm. Med. 16:141–152 (2002).
22. R. A. Anderson, K. Feathergill, X. Diao, M. Cooper, R. Kirkpatrick, P. Spear, D. P. Waller, C. Chany, G. F. Doncel, B. Herold, and L. J. Zaneveld. Evaluation of poly(styrene-4-sulfonate) as a preventive agent for conception and sexually transmitted diseases. J. Androl. 21:862–875 (2000).
23. R. A. Anderson Jr., K. Feathergill, R. Kirkpatrick, L. J. Zaneveld, K. T. Coleman, P. G. Spear, M. D. Cooper, D. P. Waller, and J. G. Thoene. Characterization of cysteamine as a potential contraceptive anti-HIV agent. J. Androl. 19:37–49 (1998).
24. M. Aulton and M. H. Abdul-Razzak. The mechanical properties of hydroxypropylmethyl cellulose films derived from aqueous systems. Part 1: The influence of plasticizers. Drug Dev. Ind. Pharm. 7:649–668 (1981).
25. S. Porter. The effect of additives on the properties of an aqueous film coating. Pharm. Tech. 4:67–75 (1980).
26. H. Schott. Polymer science. In A. Martin, P. Bustamante, and A. H. C. Chun (eds.), Physical Pharmacy (Asian Edition), B I Waverly Pvt Ltd, New Delhi, 1993, pp. 556–594.
27. S. Garg, K. Tambwekar, K. Vermani, A. Garg, C. L. Kaul, and L. J. D. Zaneveld. Compendium of pharmaceutical excipients for vaginal formulations. Pharm. Tech. 25:14–24 (2001).
28. S. Garg, K. R. Tambewekar, R. Kandarapu, K. Vermani, A. Garg, D. P. Waller, and L. J. D. Zaneveld. Development pharmaceutics of microbicide formulations. Part II. Formulation and evaluation considerations and challenges. AIDS Patient Care STDS 17:377–399 (2003).
29. E. Hardy, K. S. D. Padua, M. J. D. Osis, A. L. Jimenez, and L. J. D. Zaneveld. Women’s preferences for vaginal antimicrobial contraceptives III. Choice of a formulation, applicator, and packaging. Contraception 58:245–249 (1998).
30. S. Guzman, R. Snow, and I. Aitken. Preferences for contraceptive attributes: voices of women in Ciudad Juarez, Mexico. Int. Fam. Plann. Perspect 23:52–58 (1997).
31. D. H. Owen, J. J. Peters, and D. F. Katz. Rheological properties of contraceptive gels. Contraception 62:321–326 (2000).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Garg, S., Vermani, K., Garg, A. et al. Development and Characterization of Bioadhesive Vaginal Films of Sodium Polystyrene Sulfonate (PSS), a Novel Contraceptive Antimicrobial Agent. Pharm Res 22, 584–595 (2005). https://doi.org/10.1007/s11095-005-2490-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-005-2490-1