ABSTRACT
Purpose
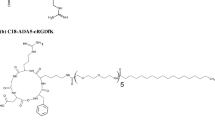
To design and synthesize fatty acid-RGD peptide amphiphiles with ADA linker for their potential delivery of hydrophobic drugs like paclitaxel targeted to αvβ3 integrin overexpressing tumors.
Methods
Four amphiphiles - C16 or C18 fatty acid–RGD peptide and ADA linker were designed and synthesized. CMC, size and zeta potential of the amphiphiles were determined. FITC loaded micelles uptake into A2058 melanoma cells was investigated at 4°C and 37°C using confocal microscopy. Paclitaxel was loaded into micelles, their encapsulation efficiency and cytotoxicity of micelles was evaluated. The stability of the micelles was determined using FRET method.
Results
Mass, 1H NMR and HPLC analysis confirmed the formation of amphiphiles and their purity. Among the amphiphiles, C18-(ADA)2-RGD amphiphile exhibited lowest CMC (9.00 ± 1.73 μM) and its micelles had suitable size (194.63 ± 44.86 nm) and zeta potential (0.27 ± 1.96 mV) for targeting. The cellular uptake of the micelles was temperature dependent and the micelles were stable. The IC50 of paclitaxel loaded in micelles decreased 50% in αvβ3 integrin overexpressing cells and showed a 4 fold increase in normal cells when compared to free paclitaxel.
Conclusion
Amphiphiles of fatty acids–ADA-RGD were synthesized. These amphiphiles formed stable micelles and were effective as targeted delivery carriers to αvβ3 integrin overexpressing tumors.












Similar content being viewed by others
REFERENCES
Shimada T, Lee S, Bates FS, Hotta A, Tirrel M. Wormlike micelle formation in peptide-lipid conjugates driven by secondary structure transformation of the headgroups. J Phys Chem B. 2009;113(42):13711–4.
Rexeisen EL, Fan W, Pangburn TO, Taribagil RR, Bates FS, Lodge TP, et al. Self-assembly of fibronectin mimetic peptide-amphiphile nanofibers. Langmuir. 2010;26(3):1953–9.
Pashuck ET, Stupp SI. Direct observation of morphological transformation from twisted ribbons into helical ribbons. J Am Chem Soc. 2010;132(26):8819–21.
Ziserman L, Lee HY, Raghavan SR, Mor A, Danino D. Unraveling the mechanism of nanotube formation by chiral self-assembly of amphiphiles. J Am Chem Soc. 2011;133(8):2511–7.
Cui H, Muraoka T, Cheetham AG, Stupp SI. Self-assembly of giant peptide nanobelts. Nano Lett. 2009;9(3):945–51.
Niece KL, Hartgerink JD, Donners JJJM, Stupp SI. Self-assembly combining two bioactive peptide-amphiphile molecules into nanofibers by electrostatic attraction. J Am Chem Soc. 2003;125(24):7146–7.
Han S, Cao S, Wang Y, Wang J, Xia D, Xu H, et al. Self-Assembly of short peptide amphiphiles: the cooperative effect of hydrophobic interaction and hydrogen bonding. Chem Eur J. 2011;17(46):13095–102.
Xu XD, Jin Y, Liu Y, Zhang XZ, Zhuo RX. Self-assembly behavior of peptide amphiphiles (PAs) with different length of hydrophobic alkyl tails. Colloids Surf B Biointerfaces. 2010;81(1):329–35.
Webber MJ, Tongers J, Renault MA, Roncalli JG, Losordo DW, Stupp SI. Development of bioactive peptide amphiphiles for therapeutic cell delivery. Acta Biomater. 2010;6(1):3–11.
Angeloni NL, Bond CW, Tang Y, Harrington DA, Zhang S, Stupp SI, et al. Regeneration of the cavernous nerve by sonic hedgehog using aligned peptide amphiphile nanofibers. Biomaterials. 2011;32(4):1091–101.
Chow LW, Wang LJ, Kaufman DB, Stupp SI. Self-assembling nanostructures to deliver angiogenic factors to pancreatic islets. Biomaterials. 2010;31(24):6154–61.
Bulut S, Erkal TS, Toksoz S, Tekinay AB, Tekinay T, Guler MO. Slow release and delivery of antisense oligonucleotide drug by self-assembled peptide amphiphile nanofibers. Biomacromolecules. 2011;12(8):3007–14.
Wiradharma N, Tong YW, Yang YY. Self-assembled oligopeptide nanostructures for co-delivery of drug and gene with synergistic therapeutic effect. Biomaterials. 2009;30(17):3100–9.
Guler MO, Claussen RC, Stupp SI. Encapsulation of pyrene within self-assembled peptide amphiphile nanofibers. J Mater Chem. 2005;15(42):4507–12.
Chen JX, Wang HY, Li C, Han K, Zhang XZ, Zhuo RX. Construction of surfactant-like tetra-tail amphiphilic peptide with RGD ligand for encapsulation of porphyrin for photodynamic therapy. Biomaterials. 2011;32(6):1678–84.
Accardo A, Tesauro D, Mangiapia G, Pedone C, Morelli G. Nanostructures by self-assembling peptide amphiphile as potential selective drug carriers. Biopol Pep Sci. 2007;88(2):115–21.
Plow EF, Haas TA, Zhang L, Loftus J, Smith JW. Ligand binding to integrins. J Biol Chem. 2000;275(29):21785–8.
Desgrosellier JS, Barnes LA, Shields DJ, Huang M, Lau SK, Prévost N, et al. An integrin alpha(v)beta(3)-c-Src oncogenic unit promotes anchorage-independence and tumor progression. Nat Med. 2009;15(10):1163–9.
Cox D, Brennan M, Moran N. Integrins as therapeutic targets: lessons and opportunities. Nat Rev Drug Discov. 2010;9(10):804–20.
Hölig P, Bach M, Völkel T, Nahde T, Hoffmann S, Müller R, et al. Novel RGD lipopeptides for the targeting of liposomes to integrin-expressing endothelial and melanoma cells. Protein Eng Des Sel. 2004;17(5):433–41.
Shen SI, Kotamraj PR, Bhattacharya S, Li X, Jasti BR. Synthesis and characterization of RGD-fatty acid amphiphilic micelles as targeted delivery carriers for anticancer agents. J Drug Target. 2007;15(1):51–8.
Antunes P, Ginj M, Walter MA, Chen J, Reubi JC, Maecke HR. Influence of different spacers on the biological profile of a DOTA-somatostatin analogue. Bioconjug Chem. 2007;18(1):84–92.
Wasserheit C, Frazein A, Oratz R, Sorich J, Downey A, Hochster H, et al. Phase II trial of paclitaxel and cisplatin in women with advanced breast cancer: an active regimen with limiting neurotoxicity. J Clin Oncol. 1996;14(7):1993–9.
Weiss RB, Donehower RC, Wiernik PH, Ohnuma T, Gralla RJ, Trump DL, et al. Hypersensitivity reactions from taxol. J Clin Oncol. 1990;8(7):1263–8.
Zhan C, Gu B, Xie C, Li J, Liu Y, Lu W. Cyclic RGD conjugated poly(ethylene glycol)-co-poly(lactic acid) micelle enhances paclitaxel anti-glioblastoma effect. J Control Release. 2010;143(1):136–42.
Hu Z, Luo F, Pan Y, Hou C, Ren L, Chen J, et al. Arg-Gly-Asp (RGD) peptide conjugated poly(lactic acid)-poly(ethylene oxide) micelle for targeted drug delivery. J Biomed Mater Res A. 2008;85(3):797–807.
Jiang X, Sha X, Xin H, Chen L, Gao X, Wang X, et al. Self-aggregated pegylated poly (trimethylene carbonate) nanoparticles decorated with c(RGDyK) peptide for targeted paclitaxel delivery to integrin-rich tumors. Biomaterials. 2011;32(35):9457–69.
Danhier F, Vroman B, Lecouturier N, Crokart N, Pourcelle V, Freichels H, et al. Targeting of tumor endothelium by RGD-grafted PLGA-nanoparticles loaded with paclitaxel. J Control Release. 2009;140(2):166–73.
Sovadinova I, Palermo EF, Huang R, Thoma LM, Kuroda K. Mechanism of polymer-induced hemolysis: nanosized pore formation and osmotic lysis. Biomacromolecules. 2011;12(1):260–8.
Kotamraj P, Russu WA, Jasti B, Wu J, Li X. Novel integrin-targeted binding-triggered drug delivery system for methotrexate. Pharm Res. 2011;28(12):3208–19.
Tang R, Ji W, Wang C. Amphiphilic block copolymers bearing ortho ester side-chains: pH-dependent hydrolysis and self-assembly in water. Macromol Biosci. 2010;10(2):192–201.
Lee ES, Oh YT, Youn YS, Nam M, Park B, Yun J, et al. Binary mixing of micelles using Pluronics for a nano-sized drug delivery system. Colloids Surf B Biointerfaces. 2011;82(1):190–5.
Domínguez A, Fernández A, González N, Iglesias E, Montenegro L. Determination of critical micelle concentration of some surfactants by three techniques. J Chem Edu. 1997;74(10):1227–31.
Danhier F, Feron O, Préat V. To exploit the tumor microenvironment: passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J Control Release. 2010;148(2):135–46.
Ding H, Yong KT, Roy I, Hu R, Wu F, Zhao L, et al. Bioconjugated PLGA-4-arm-PEG branched polymeric nanoparticles as novel tumor targeting carriers. Nanotechology. 2011;22(16):165101.
Qui LY, Bae YH. Polymer architecture and drug delivery. Pharm Res. 2006;23(1):1–30.
Wang Y, Wang X, Zhang Y, Yang S, Wang J, Zhang X, et al. RGD-modified polymeric micelles as potential carriers for targeted delivery to integrin-overexpressing tumor vasculature and tumor cells. J Drug Target. 2009;179(6):459–67.
Chu-Kung AF, Nguyen R, Bozzelli KN, Tirrell M. Chain length dependence of antimicrobial peptide-fatty acid conjugate activity. J Colloid Interface Sci. 2010;345(2):160–7.
Koppelhus U, Shiraishi T, Zachar V, Pankratova S, Nielsen PE. Improved cellular activity of antisense peptide nucleic acids by conjugation to a cationic peptide-lipid (CatLip) domain. Bioconjug Chem. 2008;19(8):1526–34.
Bellmann-Sickert K, Elling CE, Madsen AN, Little PB, Lundgren K, Gerlach LO, et al. Long-acting lipidated analogue of human pancreatic polypeptide is slowly released into circulation. J Med Chem. 2011;54(8):2658–67.
Matsonand JB, Stupp SI. Drug release from hydrazone-containing peptide amphiphiles. Chem Commun (Camb). 2011;47(28):7962–4.
Kim JK, Anderson J, Jun HW, Repka MA, Jo S. Self-assembling peptide amphiphile-based nanofiber gel for bioresponsive cisplatin delivery. Mol Pharm. 2009;6(3):978–85.
Xiong JP, Stehle T, Zhang R, Joachimiak A, Frech M, Goodman SL, et al. Crystal structure of the extracellular segment of integrin alpha Vbeta3 in complex with an Arg-Gly-Asp ligand. Science. 2002;296(5565):151–5.
Zhang H, Schneider SE, Bray BL, Friedrich PE, Tvermoes NA, Mader CJ, et al. Process development of TRI-999, a fatty-acid modified hiv fusion inhibitory peptide. Org Process Res Dev. 2008;12(1):101–10.
Adams ML, Lavasanifar A, Kwon GS. Amphiphilic block copolymers for drug delivery. J Pharm Sci. 2003;92(7):1343–55.
Lee ES, Na K, Bae YH. Polymeric micelle for tumor pH and folate-mediated targeting. J Control Release. 2003;91(1–2):103–13.
Zhang Y, Wang X, Wang J, Zhang X, Zhang Q. Octreotide-modified polymeric micelles as potential carriers for targeted docetaxel delivery to somatostatin receptor overexpressing tumor cells. Pharm Res. 2011;28(5):1167–78.
Kedar U, Phutane P, Shidhaye S, Kadam V. Advances in polymeric micelles for drug delivery and tumor targeting. Nanomedicine. 2010;6(6):714–29.
Li S, Huang L. Pharmacokinetics and biodistribution of nanoparticles. Mol Pharm. 2008;5(4):496–504.
Alexis F, Pridgen E, Molnar LK, Farokhzad OC. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol Pharm. 2008;5(4):505–15.
Castel S, Pagan R, Mitjans F, Piulats J, Goodman S, Jonczyek A, et al. RGD Peptides and monoclonal antibodies, antagonists of αv−Integrin, enter the cells by independent endocytic pathways. Lab Invest. 2001;81(12):1615–26.
Torchilin VP. Structure and design of polymeric surfactant-based drug delivery systems. J Control Release. 2001;73(2–3):137–72.
Chen H, Kim S, Li L, Wang S, Park K, Cheng JX. Release of hydrophobic molecules from polymer micelles into cell membranes revealed by förster resonance energy transfer imaging. PNAS. 2008;105(18):6596–601.
Chen H, Kim S, He W, Wang H, Low PS, Park K, et al. Fast release of lipophilic agents from circulating PEG-PDLLA micelles revealed by in vivo förster resonance energy transfer imaging. Langmuir. 2008;24(10):5213–7.
Lu J, Owen SC, Shoichet MS. Stability of self-assembled polymeric micelles in serum. Macromolecules. 2011;44(15):6002–8.
Yang C, Tan JPK, Cheng W, Attia ABE, Ting CTY, Nelson A, et al. Supramolecular nanostructures designed for high cargo loading capacity and kinetic stability. Nano Today. 2010;5(6):515–23.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 1084 kb)
Rights and permissions
About this article
Cite this article
Javali, N.M., Raj, A., Saraf, P. et al. Fatty Acid–RGD Peptide Amphiphile Micelles as Potential Paclitaxel Delivery Carriers to αvβ3Integrin Overexpressing Tumors. Pharm Res 29, 3347–3361 (2012). https://doi.org/10.1007/s11095-012-0830-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-012-0830-5