Abstract
Purpose
Chitosan microparticles containing celecoxib (CB), were developed as chemoprevention of bladder cancer. Furthermore two inclusion complexes of CB with methyl-β-cyclodextrin (C1 and C2) were prepared to improve the solubility of the drug.
Methods
C1 and C2 were obtained by freeze-drying and characterized in the solid state and in solution. Microparticles loaded with CB or C1 or C2 were prepared by spray drying and fully characterized.
Results
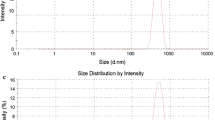
The yield and encapsulation efficiencies of microparticles depended by both the viscosity and the presence of the inclusion complex in the feed medium nebulised. Generally, the microparticles exhibited a spherical shape with mean diameter of approximately 2 μm which was compatible with local intravesical administration using a catheter. The CB release studies from the microparticles allowed us to identify both immediate release systems (microparticles including the complexes) and prolonged release systems (microparticles including CB alone). The latter exhibited good adhesion to the bladder mucosa, as highlighted by a mucoadhesion study. Histological studies revealed a desquamation of the superficial cells when the bladder mucosa was treated with microparticles loaded with CB, while the morphology of the urothelium did not change when it was treated with microparticles loaded with the inclusion complex.
Conclusion
A new CB intravesical formulation than can easily be administered with a catheter and is able to release the drug at the target site for several hours was realized. This new delivery system could be a good alternative to classic oral CB administration.





Similar content being viewed by others
Abbreviations
- 1H-NMR:
-
Proton nuclear magnetic resonance
- CB:
-
Celecoxib
- CH:
-
Chitosan
- DL:
-
Drug loading
- DSC:
-
Differential scanning calorimetr
- EE%:
-
Efficiency encapsulation percentage
- FDA:
-
Fluorescein diacetate
- FT-IR:
-
Fourier Transform infrared
- HPLC:
-
High performance liquid chromatography
- Me-β-CD:
-
Random methyl-β-cyclodextrin
- MPs:
-
Microparticles
- PBS:
-
Phosphate buffer solution
- PDI:
-
Polydispersity index
- SEM:
-
Scanning electron microscopy
References
Shariat SF, Kim JH, Ayala GE, Kho K, Wheeler TM, Lerner SP. Cyclooxygenase-2 is highly expressed in carcinoma in situ and T1 transitional cell carcinoma of the bladder. J Urol. 2003;169(3):938–42.
Fischer SM, Lo HH, Gordon GB, Seibert K, Kelloff G, Lubet RA, et al. Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, and indomethacin against ultraviolet light-induced skin carcinogenesis. Mol Carcinog. 1999;25(4):231–40.
Grubbs CJ, Lubet RA, Koki AT, Leathy KM, Masferrer JL, Steele VE, et al. Celecoxib inhibits N-butyl-N-(4-hydroxybutyl)-nitrosamine-induced urinary bladder cancers in male B6D2F1 mice and female Fischer-344 rats. Cancer Res. 2000;60(20):5599–602.
Pentland AP, Schoggins JW, Scott GA, Khan KN, Han R. Reduction of UV-induced skin tumors in hairless mice by selective COX-2 inhibition. Carcinogenesis. 1999;20(10):1939–44.
Donat SM. Evaluation and follow-up strategies for superficial bladder cancer. Urol Clin N Am. 2003;30(4):765–76.
Castelao JE, Yuan JM, Gago-Dominguez M, Yu MC, Ross RK. Non-steroidal anti-inflammatory drugs and bladder cancer prevention. Br J Cancer. 2000;82(7):1364–9.
Alshafie GA, Abou-Issa HM, Seibert K, Harris RE. Chemotherapeutic evaluation of celecoxib, a cyclooxygenase-2 inhibitor, in a rat mammary tumor model. Oncol Rep. 2000;7(6):1377–81.
Harris RE, Alshafie GA, Abou-Issa HM, Seibert K. Chemoprevention of breast cancer in rats by celecoxib, a cyclooxygenase-2 inhibitor. Cancer Res. 2000;60(8):2101–3.
Williams CS, Watson AJM, Sheng H, Helou R, Shao J, DuBois RN. Celecoxib prevents tumor growth in vivo without toxicity to normal gut: lack of correlation between in vitro and in vivo models. Cancer Res. 2000;60(21):6045–51.
Rawat S, Jain SK. Solubility enhancement of celecoxib using β-cyclodextrin inclusion complexes. Eur J Pharm Biopharm. 2004;57(2):263–7.
Sinha VR, Anitha R, Ghosh S, Nanda A, Kumria R. Complexation of celecoxib with β-cyclodextrin: characterization of the interaction in solution and in solid state. J Pharm Sci. 2005;94(3):676–87.
Nagarsenker MS, Joshi MS. Celecoxib-cyclodextrin systems: characterization and evaluation of in vitro and in vivo advantage. Drug Dev Ind Pharm. 2005;31(2):169–78.
Ventura CA, Giannone I, Paolino D, Pistarà V, Corsaro A, Puglisi G. Preparation of celecoxib-dimethyl-β-cyclodextrin inclusion complex: characterization and in vitro permeation study. Eur J Med Chem. 2005;40(7):624–31.
Kerec M, Bogataj M, Veranic P, Mrhar A. Permeability of pig urinary bladder wall: the effect of chitosan and the role of calcium. Eur J Pharm Sci. 2005;25(1):113–21.
Kerec M, Bogataj M, Mrhar A. Enhanced permeability of the urinary bladder wall: the role of polymer charge. Pharmazie. 2009;64(4):232–7.
Lopedota A, Cutrignelli A, Trapani A, Boghetich G, Denora N, Laquintana V, et al. Effects of different cyclodextrins on the morphology, loading and release properties of poly(DL-lactide-co-glycolide)-microparticles containing the hypnotic agent etizolam. J Microencapsul. 2007;24(3):214–24.
Lopedota A, Trapani A, Cutrignelli A, Laquintana V, Denora N, Franco M, et al. Effects of cyclodextrins on physico-chemical and release properties of Eudragit RS 100 microparticles containing glutathione. J Incl Phenom Macrocycl Chem. 2007;57(1):425–32.
Benita S. Microencapsulation: methods and industrial applications. New York: Marcel Dekker; 1996.
Cutrignelli A, Lopedota A, Denora N, Iacobazzi RM, Fanizza E, Laquintana V, et al. A new complex of curcumin with sulfobutylether-β-cyclodextrin: characterization studies and in vitro evaluation of cytotoxic and antioxidant activity on HepG-2 cells. J Pharm Sci. 2014;103(12):3932–40.
Higuchi T, Connors KA. Phase solubility techniques. Anal Chem Instrum. 1965;4:117–212.
Barthelmes J, Dünnhaupt S, Unterhofer S, Perera G, Schlocker W, Bernkop-Schnürch A. Thiolated particles as effective intravesical drug delivery systems for treatment of bladder-related diseases. Nanomedicine. 2013;8(1):65–75.
Barthelmes J, Perera G, Hombach J, Dünnhaupt S, Bernkop-Schnürch A. Development of a mucoadhesive nanoparticulate drug delivery system for a targeted drug release in the bladder. Int J Pharm. 2011;416(1):339–45.
Mastrodonato M, Portincasa P, Mentino D, Rossi R, Resta L, Ferri D, et al. Caveolin-1 and mitochondrial alterations in regenerating rat liver. Microsc Res Tech. 2012;75(8):1026–32.
Lopedota A, Cutrignelli A, Denora N, Laquintana V, Lopalco A, Selva S, et al. New ethanol and propylene glycol free gel formulations containing a minoxidil-methyl-β-cyclodextrin complex as promising tools for alopecia treatment. Drug Dev Ind Pharm. 2015;41(5):728–36.
Ventura CA, Tommasini S, Falcone A, Giannone I, Paolino D, Sdrafkakis V, et al. Influence of modified cyclodextrins on solubility and percutaneous absorption of celecoxib through human skin. Int J Pharm. 2006;314(1):37–45.
Ventura CA, Trendi S, Puglisi G, Bousquet E, Panza L. Improvement of water solubility and dissolution rate of ursodeoxycholic acid and chenodeoxycholic acid by complexation with natural and modified beta-cyclodextrins. Int J Pharm. 1997;149(1):1–13.
Uekama K, Hirayama F, Irie T. Applications of cyclodextrins. In: Debeor ABG, editor. Drug absorption enhancement: concepts, possibilities, limitations and trends. London: Harwood Academic Publishers; 1997. p. 411–56.
Kotze AF, Luessen HI, de Leeuw BJ, de Boer BG, Verhoef JC, Junginger HE. Comparison of the effect of different chitosan salts and N-trimethyl chitosan chloride on the permeability of intestinal epithelial cells (Caco-2). J Control Release. 1998;51(1):35–46.
Artusson P, Lindmark T, Davis S, Illum L. Effect of chitosan on the permeability of monolayers of intestinal epithelial cells (Caco-2). Pharm Res. 1994;11(9):1358–61.
Aspden TJ, Skaugrud O, Illum L. Chitosan as a nasal delivery system: evaluation of insulin absorption enhancement and effect on nasal membrane integrity using rat models. Eur J Pharm Sci. 1996;4(1):23–31.
Palmieri GF, Wehrlè P, Stamm A. Evaluation of spray-drying as a method to prepare microparticles for controlled drug release. Drug Dev Ind Pharm. 1994;20(18):2859–79.
He P, Davis SS, Illum L. Chitosan microspheres prepared by spray drying. Int J Pharm. 1999;187(1):53–65.
Prinn KB, Costantino HR, Tracy M. Statistical modeling of protein spray drying at the lab scale. AAPS PharmSciTech. 2002;3(1), E4.
Cervera MF, Heinämäki J, de la Paz N, López O, Maunu SL, Virtanen T, et al. Effects of spray drying on physicochemical properties of chitosan acid salts. AAPS PharmSciTech. 2011;12(2):637–49.
Nunthanid J, Laungtana-Anan M, Sriamornsak P, Limmatvapirat S, Puttipipatkhachorn S, Lim L, et al. Characterization of chitosan acetate as a binder for sustained release tablets. J Control Release. 2004;99(1):15–26.
Albrecht K, Greindl M, Kremser C, Wolf C, Debbage P, Bernkop-Schnurch A. Comparative in vivo mucoadhesion studies of thiomer formulations using magnetic resonance imaging and fluorescence detection. J Control Release. 2006;115:78–84.
Albrecht K, Zirm EJ, Palmberger TF, Schlocker W, Bernkop-Schnurch A. Preparation of thiomer microparticles and in vitro evaluation of parameters influencing their mucoadhesive properties. Drug Dev Ind Pharm. 2006;32:1149–57.
ACKNOWLEDGMENTS AND DISCLOSURES
The authors acknowledge the financial support of the University of Bari (Italy), and the Inter-University Consortium for Research on the Chemistry of Metal Ions in Biological Systems (C.I.R.C.M.S.B.). We thank Mr. Antonio Palermo for his skilful technical assistance and Dr.Vito Masciopinto for providing us the pig bladders that were used for the mucoadhesion study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 65 kb)
Rights and permissions
About this article
Cite this article
Lopedota, A., Cutrignelli, A., Laquintana, V. et al. Spray Dried Chitosan Microparticles for Intravesical Delivery of Celecoxib: Preparation and Characterization. Pharm Res 33, 2195–2208 (2016). https://doi.org/10.1007/s11095-016-1956-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-016-1956-7