Abstract
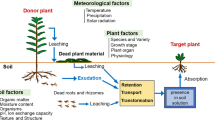
We investigated the roles of Al-binding ligands in Al exclusion from roots and in internal Al detoxification in roots as Al resistance mechanisms in two Al-resistant Myrtaceae trees, Eucalyptus camaldulensis Dehnh. and Melaleuca cajuputi Powell. The amounts of ligands secreted from roots and contained in root tips of these species were compared with those of an Al-sensitive species, Melaleuca bracteata F. Muell., after the roots were exposed to 0 or 1 mM AlCl3 solution. Secretion of well-known ligands (citrate, oxalate, and malate) from roots under Al treatment was low in all species. However, in E. camaldulensis, the Al-binding capacity of root exudates under Al treatment was considerable and was higher than that in M. bracteata. Gel filtration chromatography revealed that a low-molecular-weight Al-binding ligand was secreted from roots in response to Al only in E. camaldulensis. On the other hand, the Al-binding capacity of cell sap in root tips under Al treatment was similar for the resistant and sensitive species. These results suggest that Al exclusion by secretion of the unknown low-molecular-weight Al-binding ligand from roots contributes to the Al resistance of E. camaldulensis, whereas M. cajuputi has developed Al-resistance mechanisms other than secretion of ligands from roots or concentration of internal ligands in root tips.







Similar content being viewed by others
References
Barceló J, Poschenrieder C (2002) Fast root growth responses, root exudates, and internal detoxification as clues to the mechanisms of aluminium toxicity and resistance: a review. Environ Exp Bot 48:75–92
Basu U, Godbold D, Taylor GJ (1994) Aluminum resistance in Triticum aestivum associated with enhanced exudation of malate. J Plant Physiol 144:747–753
Basu U, Good AG, Aung T, Slaski JJ, Basu A, Briggs KG, Taylor GJ (1999) A 23-kDa, root exudate polypeptide co-segregates with aluminum resistance in Triticum aestivum. Physiol Plant 106:53–61
Degenhardt J, Larsen PB, Howell SH, Kochian LV (1998) Aluminum resistance in the Arabidopsis mutant alr-104 is caused by an aluminum-induced increase in rhizosphere pH. Plant Physiol 117:19–27
Delhaize E, Ryan PR (1995) Aluminum toxicity and tolerance in plants. Plant Physiol 107:315–321
Dixon RA, Paiva NL (1995) Stress-induced phenylpropanoid metabolism. Plant Cell 7:1085–1097
Eticha D, Stass A, Horst WJ (2005) Cell-wall pectin and its degree of methylation in the maize root-apex: significance for genotypic differences in aluminium resistance. Plant Cell Environ 28:1410–1420
Heim A, Luster J, Brunner I, Frey B, Frossard E (1999) Effects of aluminium treatment on Norway spruce roots: Aluminium binding forms, element distribution, and release of organic substances. Plant Soil 216:103–116
Kidd PS, Llugany M, Poschenrieder C, Gunsé B, Barceló J (2001) The role of root exudates in aluminium resistance and silicon-induced amelioration of aluminium toxicity in three varieties of maize (Zea mays L.). J Exp Bot 52:1339–1352
Kinraide TB (1991) Identity of the rhizotoxic aluminium species. Plant Soil 134:167–178
Kinraide TB (2006) Plasma membrane surface potential (ΨPM) as a determinant of ion bioavailability: a critical analysis of new and published toxicological studies and a simple method for the computation of plant ψPM. Environ Toxicol Chem 25:3188–3198
Kochian LV (1995) Cellular mechanisms of aluminum toxicity and resistance in plants. Annu Rev Plant Physiol Plant Mol Biol 46:237–260
Kochian LV, Hoekenga OA, Piñeros MA (2004) How do crop plants tolerate acid soils? Mechanisms of aluminum tolerance and phosphorous efficiency. Annu Rev Plant Biol 55:459–493
Ma JF, Hiradate S (2000) Form of aluminium for uptake and translocation in buckwheat (Fagopyrum esculentum Moench). Planta 211:355–360
Ma JF, Hiradate S, Nomoto K, Iwashita T, Matsumoto H (1997) Internal detoxification mechanism of Al in hydrangea. Identification of Al form in the leaves. Plant Physiol 113:1033–1039
Ma JF, Hiradate S, Matsumoto H (1998) High aluminium resistance in buckwheat: II. Oxalic acid detoxifies aluminium internally. Plant Physiol 117:753–759
Ma JF, Ryan PR, Delhaize E (2001) Aluminium tolerance in plants and the complexing role of organic acids. Trends Plant Sci 6:273–278
Matsumoto H (2000) Cell biology of aluminum toxicity and tolerance in higher plants. Int Rev Cytol 200:1–46
Menzies NW, Kerven GL, Bell LC, Edwards DG (1992) Determination of total soluble aluminum in soil solution using pyrocatechol violet, lanthanum and iron to discriminate against micro-particulates and organic ligands. Commun Soil Sci Plant Anal 23:2525–2545
Nguyen NT, Nakabayashi K, Thompson J, Fujita K (2003) Role of exudation of organic acids and phosphate in aluminum tolerance of four tropical woody species. Tree Physiol 23:1041–1050
Ofei-Manu P, Wagatsuma T, Ishikawa S, Tawaraya K (2001) The plasma membrane strength of the root-tip cells and root phenolic compounds are correlated with Al tolerance in several common woody plants. Soil Sci Plant Nutr 47:359–375
Osaki M, Watanabe T, Tadano T (1997) Beneficial effects of aluminum on growth of plants adapted to low pH soils. Soil Sci Plant Nutr 43:551–563
Osaki M, Watanabe T, Ishizawa T, Nilnond C, Nuyim T, Sittibush C, Tadano T (1998) Nutritional characteristics in leaves of native plants grown in acid sulfate, peat, sandy podzolic, and saline soils distributed in Peninsular Thailand. Plant Soil 201:175–182
Osawa H, Kojima K (2006) Citrate-release-mediated aluminum resistance is coupled to the inducible expression of mitochondrial citrate synthase gene in Paraserianthes falcataria. Tree Physiol 26:565–574
Pellet DM, Papernik LA, Kochian LV (1996) Multiple aluminum-resistance mechanisms in wheat: roles of root apical phosphate and malate exudation. Plant Physiol 112:591–597
Piñeros MA, Shaff JE, Manslank HS, Alves VMC, Kochian LV (2005) Aluminum resistance in maize cannot be solely explained by root organic acid exudation. A comparative physiological study. Plant Physiol 137:231–241
Shen RF, Ma JF, Kyo M, Iwashita T (2002) Compartmentation of aluminium in leaves of an Al-accumulator, Fagopyrum esculentum Moench. Planta 215:394–398
Silva IR, Smyth TJ, Raper D, Carter TE, Rufty TW (2001) Differential aluminum tolerance in soybean: An evaluation of the role of organic acids. Physiol Plant 112:200–210
Swain T, Hillis WE (1959) The phenolic constituents of Prunus domestica. I. The quantitative analysis of phenolic constituents. J Sci Food Agric 10:63–68
Tahara K, Norisada M, Hogetsu T, Kojima K (2005a) Aluminum tolerance and aluminum-induced deposition of callose and lignin in the root tips of Melaleuca and Eucalyptus species. J For Res 10:325–333
Tahara K, Norisada M, Tange T, Yagi H, Kojima K (2005b) Ectomycorrhizal association enhances Al tolerance by inducing citrate secretion in Pinus densiflora. Soil Sci Plant Nutr 51:397–403
van Breemen N, Pons LJ (1978) Acid sulfate soils and rice. In Soils and Rice. Ed. International Rice Research Institute, Los Baños, pp 739–761
Vance GF, Stevenson FJ, Sikora FJ (1996) Environmental chemistry of aluminum-organic acid complexes. In: Sposito G (ed) In the environmental chemistry of aluminum 2nd. edn. Lewis Publishers, Boca Raton. pp 169–220
Wagatsuma T, Khan MSH, Rao IM, Wenzl P, Tawaraya K, Yamamoto T, Kawamura T, Hosogoe K, Ishikawa S (2005) Methylene blue stainability of root-tip protoplasts as an indicator of aluminum tolerance in a wide range of plant species, cultivars and lines. Soil Sci Plant Nutr 51:991–998
Weiss M, Mikolajewski S, Peipp H, Schmitt U, Schmidt J, Wray V, Strack D (1997) Tissue-specific and development-dependent accumulation of phenylpropanoids in larch mycorrhizas. Plant Physiol 114:15–27
Wenzl P, Patiño GM, Chaves AL, Mayer JE, Rao IM (2001) The high level of aluminum resistance in signalgrass is not associated with known mechanisms of external aluminum detoxification in root apices. Plant Physiol 125:1473–1484
Wenzl P, Chaves AL, Patiño GM, Mayer JE, Rao IM (2002) Aluminum stress stimulates the accumulation of organic acids in root apices of Brachiaria species. J Plant Nutr Soil Sci 165:582–588
Yamanoshita T, Masumori M, Yagi H, Kojima K (2005) Effects of flooding on downstream processes of glycolysis and fermentation in roots of Melaleuca cajuputi seedlings. J For Res 10:199–204
Zheng SJ, Ma JF, Matsumoto H (1998) High aluminium resistance in buckwheat: I. Al-induced specific secretion of oxalic acid from root tips. Plant Physiol 117:745–751
Zheng SJ, Yang JL, He YF, Yu XH, Zhang L, You JF, Shen RF, Matsumoto H (2005) Immobilization of aluminum with phosphorus in roots is associated with high aluminum resistance in buckwheat. Plant Physiol 138:297–303
Acknowledgments
We thank Dr. Kenji Iiyama and Dr. Kyoko Katsumata of the University of Tokyo for their suggestions on the gel filtration chromatography. We are grateful to Dr. Hiroki Osawa of the University of Tokyo for his critical review of the manuscript. This study was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan and by the Global Environment Research Fund from the Ministry of the Environment of Japan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Thomas B. Kinraide.
Rights and permissions
About this article
Cite this article
Tahara, K., Norisada, M., Yamanoshita, T. et al. Role of aluminum-binding ligands in aluminum resistance of Eucalyptus camaldulensis and Melaleuca cajuputi . Plant Soil 302, 175–187 (2008). https://doi.org/10.1007/s11104-007-9464-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-007-9464-5