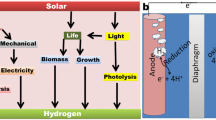
Abstract
Global energy demand is increasing rapidly and due to intensive consumption of different forms of fuels, there are increasing concerns over the reduction in readily available conventional energy resources. Because of the deleterious atmospheric effects of fossil fuels and the uncertainties of future energy supplies, there is a surge of interest to find environmentally friendly alternative energy sources. Hydrogen (H2) has attracted worldwide attention as a secondary energy carrier, since it is the lightest carbon-neutral fuel rich in energy per unit mass and easy to store. Several methods and technologies have been developed for H2 production, but none of them are able to replace the traditional combustion fuel used in automobiles so far. Extensively modified and renovated methods and technologies are required to introduce H2 as an alternative efficient, clean, and cost-effective future fuel. Among several emerging renewable energy technologies, photobiological H2 production by oxygenic photosynthetic microbes such as green algae and cyanobacteria or by artificial photosynthesis has attracted significant interest. In this short review, we summarize the recent progress and challenges in H2-based energy production by means of biological and artificial photosynthesis routes.









Similar content being viewed by others
Abbreviations
- Chl:
-
Chlorophyll
- CO2 :
-
Carbon dioxide
- CH4 :
-
Methane
- FdOX :
-
Oxidized form of ferredoxin
- FdRED :
-
Reduced form of ferredoxin
- H2 :
-
Hydrogen
- PS I and PS II:
-
Photosystem I and photosystem II
- LHCs:
-
Light-harvesting complexes
- NGS:
-
Natural gas reformation reaction
- HTWS:
-
High-temperature thermochemical water splitting
- NHTE:
-
Nuclear high-temperature electrolysis
- Nm3 :
-
Normal cubic meter
- TEM:
-
Transmission electron microscopy
- HRTEM:
-
High-resolution transmission electron microscopy
- WOC:
-
Water-oxidizing complex
References
Abraham S (2002) Toward a more secure and cleaner energy future for America: national hydrogen energy roadmap; production, delivery, storage, conversion, applications, public education and outreach. US Department Energy, Washington
Adamson AW, Demas JN (1971) New photosensitizer. Tris (2, 2′-bipyridine) ruthenium (II) chloride. J Am Chem Soc 93:1800–1801
Allakhverdiev SI (2012) Photosynthetic and biomimetic hydrogen production. Int J Hydrog Energy 37:8744–8752
Allakhverdiev SI, Kreslavski VD, Thavasi V, Zharmukhamedov SK, Klimov VV, Nagata T, Nishihara H, Ramakrishna S (2009) Hydrogen photoproduction by use of photosynthetic organisms and biomimetic systems. Photochem Photobiol Sci 8:148–156
Allakhverdiev SI, Thavasi V, Kreslavski VD, Zharmukhamedov SK, Klimov VV, Ramakrishna S, Los DA, Mimuro M, Nishihara H, Carpentier R (2010a) Photosynthetic hydrogen production. J Photochem Photobiol C 11:87–99
Allakhverdiev SI, Kreslavski VD, Thavasi V, Zharmukhamedov SK, Klimov VV, Nishihara H, Ramakrishna S, Mimuro M, Carpentier R, Nagata T (2010b) Photosynthetic energy conversion: hydrogen photoproduction by natural and biomimetic systems. In: Mukherjee Amitava (ed) Biomimetics, learning from nature. IN-TECH, Vukovar, pp 49–76
Allen JF (1975) Oxygen reduction and optimum production of ATP in photosynthesis. Nature 256:599–600. doi:10.1038/256599a0
Araújo WL, Nunes-Nesi A, Fernie AR (2014) On the role of plant mitochondrial metabolism and its impact on photosynthesis in both optimal and sub-optimal growth conditions. Photosynth Res 119:141–156
Arnon DI (1959) Conversion of light into chemical energy in photosynthesis. Nature 184:10–20
Azwar MY, Hussain MA, Abdul-Wahab AK (2014) Development of biohydrogen production by photobiological, fermentation and electrochemical processes: a review. Renew Sustain Energy Rev 31:158–173
Baker NR (2008) Chlorophyll fluorescence: a Probe of Photosynthesis in vivo. Annu Rev Plant Biol 59:89–113
Bandyopadhyay A, Stöckel J, Min H et al (2010) High rates of photobiological H2 production by a cyanobacterium under aerobic conditions. Nat Commun 1:139
Björkman O, Demmig-Adams B (1995) Regulation of photosynthetic light energy capture, conversion, and dissipation in leaves of higher plants. Ecophysiology photosynthesis. Springer, Berlin, pp 17–47
Björn LO, Papageorgiou GC, Blankenship RE, Govindjee (2009) A viewpoint: Why chlorophyll a? Photosynth Res 99:85–98. doi:10.1007/s11120-008-9395-x
Chou LY, Liu R, He W, Geh N, Lin Y, Hou EYF, Wang D, Hou HJM (2012) Direct oxygen and hydrogen production by water splitting using a robust bioinspired manganese-oxo oligomer complex/tungsten oxide catalytic system. Int J Hydrog Energy 37:8889–8896
Ciamician G (1912) The photochemistry of the future. Science 36:385–394
Concepcion JJ, Jurss JW, Templeton JL, Meyer TJ (2008) Mediator-assisted water oxidation by the ruthenium “blue dimer” cis, cis-[(bpy) 2 (H2O) RuORu (OH2)(bpy) 2] 4+. Proc Natl Acad Sci 105:17632–17635
Eberhard S, Finazzi G, Wollman F-A (2008) The dynamics of photosynthesis. Annu Rev Genet 42:463–515. doi:10.1146/annurev.genet.42.110807.091452
Esper B, Badura A, Rögner M (2006) Photosynthesis as a power supply for (bio-) hydrogen production. Trends Plant Sci 11:543–549
Ewan BCR, Allen RWK (2005) A figure of merit assessment of the routes to hydrogen. Int J Hydrog Energy 30:809–819
Ferreira KN, Iverson TM, Maghlaoui K et al (2004) Architecture of the photosynthetic oxygen-evolving center. Science 303:1831–1838
Flexas J, Briantais J-M, Cerovic Z et al (2000) Steady-state and maximum chlorophyll fluorescence responses to water stress in grapevine leaves: a new remote sensing system. Remote Sens Environ 73:283–297
Flexas J, Escalona JM, Evain S et al (2002) Steady-state chlorophyll fluorescence (Fs) measurements as a tool to follow variations of net CO2 assimilation and stomatal conductance during water-stress in C3 plants. Physiol Plant 114:231–240
Freedman A, Cavender-Bares J, Kebabian PL et al (2002) Remote sensing of solar-excited plant fluorescence as a measure of photosynthetic rate. Photosynthetica 40:127–132
Gaffron H (1939) Reduction of CO2 with H2 in green plants. Nature 143:204–205
Gafney HD, Adamson AW (1972) Excited state Ru (bipyr) 32 + as an electron-transfer reductant. J Am Chem Soc 94:8238–8239
Garbulsky MF, Filella I, Verger A, Peñuelas J (2013) Photosynthetic light use efficiency from satellite sensors: From global to Mediterranean vegetation. Environ. Exp, Bot
Gust D, Moore TA, Moore AL (2009) Solar fuels via artificial photosynthesis. Acc Chem Res 42:1890–1898
Hemschemeier A, Melis A, Happe T (2009) Analytical approaches to photobiological hydrogen production in unicellular green algae. Photosynth Res 102:523–540
Huner NPA, Öquist G, Sarhan F (1998) Energy balance and acclimation to light and cold. Trends Plant Sci 3:224–230
Imahori H, Mori Y, Matano Y (2003) Nanostructured artificial photosynthesis. J Photochem Photobiol C 4:51–83
Iyer A, Del-Pilar J, Kingondu CK et al (2012) Water oxidation catalysis using amorphous manganese oxides, octahedral molecular sieves (OMS-2), and octahedral layered (OL-1) manganese oxide structures. J Phys Chem C 116:6474–6483
Kotay SM, Das D (2008) Biohydrogen as a renewable energy resource-prospects and potentials. Int J Hydrog Energy 33:258–263
Limburg J, Vrettos JS, Liable-Sands LM, Rheingold AL, Crabtree RH, Brudvig GW (1999) A functional model for O–O bond formation by the O2-evolving complex in photosystem II. Science 283:1524–1527
Lynd LR, Larson E, Greene N et al (2009) The role of biomass in America’s energy future: framing the analysis. Biofuels Bioprod Biorefining 3:113–123
Maitra U, Lingampalli SR, Rao CNR (2014) Artificial photosynthesis and the splitting of water to generate hydrogen. Curr Sci 106:518–527
Maness P-C, Yu J, Eckert C, Ghirardi ML (2009) Photobiological hydrogen production—prospects and challenges. Microbe Magazine 4(6):275–280
Maurino VG, Weber APM (2013) Engineering photosynthesis in plants and synthetic microorganisms. J Exp Bot. doi:10.1093/jxb/ers263
Maxwell K (2000) Chlorophyll fluorescence–a practical guide. J Exp Bot 51:659–668
Melis A, Happe T (2001) Hydrogen production. Green algae as a source of energy. Plant Physiol 127:740–748
Müller P, Li X-P, Niyogi KK (2001) Non-photochemical quenching. A response to excess light energy. Plant Physiol 125:1558–1566
Najafpour MM (2011) Hollandite as a functional and structural model for the biological water oxidizing complex: manganese-calcium oxide minerals as a possible evolutionary origin for the camn4 cluster of the biological water oxidizing complex. Geomicrobiol J 28:714–718
Najafpour MM, Allakhverdiev SI (2012) Manganese compounds as water oxidizing catalysts for hydrogen production via water splitting: from manganese complexes to nano-sized manganese oxides. Int J Hydrog Energy 37:8753–8764
Najafpour MM, Tabrizi AM, Cecil K (2013) Nano-size layered manganese–calcium oxide as an efficient and biomimetic catalyst for water oxidation under acidic conditions: comparable to platinum. Dalton Trans 42:5085–5091
Najafpour MM, Ghobadi MZ, Sedigh DJ, Haghighi B (2014a) Nano-sized layered manganese oxide in a poly-l-glutamic acid matrix: a biomimetic, homogenized, heterogeneous structural model for the water-oxidizing complex in photosystem II. RSC Adv 4:39077–39081
Najafpour MM, Isaloo MA, Eaton-Rye JJ, Tomo T, Nishihara H, Satoh K, Carpentier R, Shen JR, Allakhverdiev SI (2014b) Water exchange in manganese-based water-oxidizing catalysts in photosynthetic systems: from the water-oxidizing complex in photosystem II to nano-sized manganese oxides. Biochim Biophys Acta 1837(9):1395–1410
Nam YS, Magyar AP, Lee D et al (2010) Biologically templated photocatalytic nanostructures for sustained light-driven water oxidation. Nat Nanotechnol 5:340–344
Nath K, Elizabeth J, Poudyal RS et al (2013a) Mobilization of photosystem II-light harvesting complex II supercomplexes during high light illumination and state transitions. Rapid Commun Photosci 2:18–23. doi:10.5857/RCP.2013.2.1.018
Nath K, Phee B-K, Jeong S et al (2013b) Age-dependent changes in the functions and compositions of photosynthetic complexes in the thylakoid membranes of Arabidopsis thaliana. Photosynth Res 117:547–556
Nocera DG (2012) The artificial leaf. Acc Chem Res 45:767–776
Pena MA, Gómez JP, Fierro JLG (1996) New catalytic routes for syngas and hydrogen production. Appl Catal A 144:7–57
Porcar-Castell A (2011) A high-resolution portrait of the annual dynamics of photochemical and non-photochemical quenching in needles of Pinus sylvestris. Physiol Plant 143:139–153
Rascher U, Damm A, van der Linden S et al (2010) Sensing of photosynthetic activity of crops. Precision crop protection challenge use heterogeneity. Springer, Netherlands, pp 87–99
Rathmann R, Szklo A, Schaeffer R (2010) Land use competition for production of food and liquid biofuels: an analysis of the arguments in the current debate. Renew Energy 35:14–22
Rathmann R, Szklo A, Schaeffer R (2012) Targets and results of the Brazilian biodiesel incentive program-has it reached the promised land? Appl Energy 97:91–100
Rey FE, Heiniger EK, Harwood CS (2007) Redirection of metabolism for biological hydrogen production. Appl Environ Microbiol 73:1665–1671
Rostrup-Nielsen JR (1984) Sulfur-passivated nickel catalysts for carbon-free steam reforming of methane. J Catal 85:31–43
Schütz K, Happe T, Troshina O et al (2004) Cyanobacterial H2 production-a comparative analysis. Planta 218:350–359
Searchinger T, Heimlich R, Houghton RA et al (2008) Use of US croplands for biofuels increases greenhouse gases through emissions from land-use change. Science 319:1238–1240
Soukupová J, Cséfalvay L, Urban O et al (2008) Annual variation of the steady-state chlorophyll fluorescence emission of evergreen plants in temperate zone. Funct Plant Biol 35:63–76
Suga M, Akita F, Hirata K et al (2015) Native structure of photosystem II at 1.95 A resolution viewed by femtosecond X-ray pulses. Nature 517:99–103
Umena Y, Kawakami K, Shen J-R, Kamiya N (2011) Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å. Nature 473:55–60
Whitmarsh J (1999) The photosynthetic process. Concepts photobiology. Springer, Netherlands, pp 11–51
Williams CR, Bees MA (2014) Mechanistic modeling of sulfur-deprived photosynthesis and hydrogen production in suspensions of chlamydomonas reinhardtii. Biotechnol Bioeng 111:320–335
Zarco-Tejada PJ, Pushnik JC, Dobrowski S, Ustin SL (2003) Steady-state chlorophyll a fluorescence detection from canopy derivative reflectance and double-peak red-edge effects. Remote Sens Environ 84:283–294
Acknowledgments
MMN is grateful to the Institute for Advanced Studies in Basic Sciences, and the National Elite Foundation for financial support. SEB is grateful to Young Researchers and Elite Club for financial support. ET was supported by Academy of Finland and Nordic Energy Research (Aquafeed project). HGN was supported by Institute for Basic Sciences (IBS-R013-D1-2015-a00), Korea. This work was also supported by a grant-in-aid for Specially Promoted Research No. 24000018 from JSPS, MEXT (Japan) to JRS, and by a grant from the Russian Science Foundation (No: 14-14-00039) to SIA.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nath, K., Najafpour, M.M., Voloshin, R.A. et al. Photobiological hydrogen production and artificial photosynthesis for clean energy: from bio to nanotechnologies. Photosynth Res 126, 237–247 (2015). https://doi.org/10.1007/s11120-015-0139-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-015-0139-4