Abstract
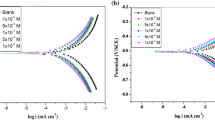
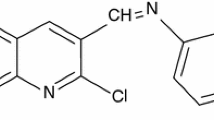
Corrosion inhibition of mild steel (MS) by chloroquine (CQ) in 1 M HCl was investigated using weight loss, polarization, electrochemical impedance spectroscopy (EIS) and quantum chemical techniques. The inhibitor showed 99 % inhibition efficiency at concentration of 3.1 × 10−4 M. Polarization studies showed that CQ is a mixed-type inhibitor. Adsorption of inhibitor molecules on the MS surface showed Langmuir adsorption isotherm. Thermodynamic parameters led to the conclusion that adsorption is predominantly chemisorption. Quantum chemical calculations were carried out to investigate the corrosion-inhibiting property of CQ. Various parameters such as energy of the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO), softness of molecule, Mullikan charges on various atoms and number of electrons transferred from inhibitor molecule to metal were calculated and correlated with the inhibiting property of CQ.







Similar content being viewed by others
References
M. Lagrenee, B. Mernari, M. Bouanis, M. Traisnel, F. Bentiss, Study of the mechanism and inhibiting efficiency of 3,5-bis(4-methylthiophenyl)-4H-1,2,4-triazole on mild steel corrosion in acidic media. Corros. Sci. 44, 573 (2002)
M. Abdallah, H.E. Megahed, A.M. Atia, Pyridine derivatives as corrosion inhibitors for dissolution of iron electrode in tartaric acid. J. Electrochem. Soc. India 35, 47 (1998)
M.A. Quraishi, M.Z.A. Rafiquee, S. Khan, N. Saxena, J. Appl. Electrochem. 37, 1153 (2007)
F. Bentiss, M. Lebrini, H. Vezin, M. Lagrenee, Mater. Chem. Phys. 87, 18 (2004)
A. Popova, E. Sokolova, S. Raicheva, M. Christov, AC and DC study of the temperature effect on mild steel corrosion in acid media in the presence of benzimidazole derivatives. Corros. Sci. 45, 33 (2003)
A.K. Singh, M.A. Quraishi, Investigation of the effect of disulfiram on corrosion of mild steel in hydrochloric acid solution. Corros. Sci. 53, 1288 (2011)
A.K. Singh, M.A. Quraishi, Adsorption properties and inhibition of mild steel corrosion in hydrochloric acid solution by ceftobiprole. J. Appl. Electrochem. 41, 7–18 (2011)
A.K. Singh, M.A. Quraishi, E.E. Ebenso, Inhibitive effect of cefuroxime on the corrosion of mild steel in hydrochloric acid solution. Int. J. Electrochem. Sci. 6, 5676 (2011)
A.K. Singh, M.A. Quraishi, Effect of cefazolin on the corrosion of mild steel in HCl solution. Corros. Sci. 52, 152 (2010)
A.K. Singh, E.E. Ebenso, M.A. Quraishi, Adsorption behaviour of cefapirin on mild steel in hydrochloric acid solution. Int. J. Electrochem. Sci. 7, 2320 (2012)
A.K. Singh, M.A. Quraishi, Inhibitive effect of diethylcarbamazine on the corrosion of mild steel in hydrochloric acid, Corros. Sci. 52, 1529 (2010)
A.K. Singh, E.E. Ebenso, Effect of ceftezole on the corrosion of mild steel in HCl solution. Int. J. Electrochem. Sci. 7, 2349 (2012)
G. Moretti, F. Guidi, G. Grion, Tryptamine as a green iron corrosion inhibitor in 0.5 M deaerated sulphuric acid. Corros. Sci. 46, 387 (2004)
F.C. Giacomelli, C. Giacomelli, M.F. Amadori, V. Schmidt, A. Spinelli, Inhibitor effect of succinic acid on the corrosion resistance of mild steel: electrochemical, gravimetric and optical microscopic studies. Mater. Chem. Phys. 83, 124 (2004)
E.S. Ferreira, C. Giacomelli, F.C. Giacomelli, A. Spinelli, Evaluation of the inhibitor effect of l-ascorbic acid on the corrosion of mild steel. Mater. Chem. Phys. 83, 129 (2004)
E.E.F. El Sherbini, Sulphamethoxazole as an effective inhibitor for the corrosion of mild steel in 1.0 M HCl solution. Mater. Chem. Phys. 61, 223 (1999)
M.S. Morad, Inhibition of iron corrosion in acid solutions by cefatrexyl: behaviour near and at the corrosion potential. Corros. Sci. 50, 436 (2008)
M. Abdallah, Antibacterial drugs as corrosion inhibitors for corrosion of aluminium in hydrochloric solution. Corros. Sci. 4(6), 2004 (1981)
S. Martinez, M.M. Huksovic, A nonlinear kinetic model introduced for the corrosion inhibitive properties of some organic inhibitors. J. Appl. Elecrochem. 33, 1137 (2003)
A.K. Singh, M.A. Quraishi, Study of some bidentate schiff bases of isatin as corrosion inhibitors for mild steel in hydrochloric acid solution. Int. J. Electrochem. Sci. 7, 3222 (2012)
C. Deslouis, B. Tribollet, G. Mengoli, M.M. Musiani, Electrochemical behaviour of copper in neutral aerated chloride solution. I. Steady-state investigation. J. Appl. Electrochem. 18, 374 (1988)
A.K. Singh, M.A. Quraishi, Inhibiting effects of 5-substituted isatin-based Mannich bases on the corrosion of mild steel in hydrochloric acid solution. J. Appl. Electrochem. 40, 1293 (2010)
A.K. Singh, M.A. Quraishi, The effect of some bis-thiadiazole derivatives on the corrosion of mild steel in hydrochloric acid. Corros. Sci. 52, 1373 (2010)
V.P. Singh, P. Singh, A.K. Singh, Synthesis, structural and corrosion inhibition studies on cobalt(II), nickel(II), copper(II) and zinc(II) complexes with 2-acetylthiophene benzoylhydrazone. Inorg. Chim. Acta 379, 56 (2011)
A.K. Singh, Inhibition of mild steel corrosion in hydrochloric acid solution by 3-(4-((Z)-indolin-3-ylideneamino)phenylimino)indolin-2-one. Ind. Eng. Chem. Res. 51, 3215 (2012)
A.K. Singh, M.A. Quraishi, Investigation of adsorption of isoniazid derivatives at mild steel/hydrochloric acid interface: electrochemical and weight loss methods. Mater. Chem. Phys. 123, 666 (2010)
A.K. Singh, S.K. Shukla, M. Singh, M.A. Quraishi, Inhibitive effect of ceftazidime on corrosion of mild steel in hydrochloric acid solution. Mater. Chem. Phys. 129, 68 (2011)
M.A. Quraishi, A. Singh, V. Singh, D.K. Yadav, A.K. Singh, Green approach to corrosion inhibition of mild steel in hydrochloric acid and sulphuric acid solutions by the extract of Murraya koenigii leaves. Mater. Chem. Phys. 122, 114 (2010)
J. Flis, T. Zakroczymski, Impedance study of reinforcing steel in simulated pore solution with tannin. J. Electrochem. Soc. 143, 2458 (1996)
M. Stern, A.L. Geary, Electrochemical polarization. J. Electrochem. Soc. 104, 56 (1957)
G.K. Gomma, M.H. Wahdan, Schiff bases as corrosion inhibitors for aluminium in hydrochloric acid solution. Mater. Chem. Phys. 39, 209 (1955)
M. Abdallah, Rhodanine azosulpha drugs as corrosion inhibitors for corrosion of 304 stainless steel in hydrochloric acid solution. Corros. Sci. 44, 717 (2002)
A.K. Singh, S.K. Shukla, M.A. Quraishi, E.E. Ebenso, Investigation of adsorption characteristics of N,N′-[(methylimino)dimethylidyne]di-2,4-xylidine as corrosion inhibitor at mild steel/sulphuric acid interface. J. Taiwan Inst. Chem. Eng. (2011). doi:10.1016/j.jtice.2011.10.012
B. Donnely, T.C. Downie, R. Grzeskowiak, H.R. Hamburg, D. Short, The effect of electronic delocalization in organic groups R in substituted thiocarbamoyl RCSNH2 and related compounds on inhibition efficiency. Corros. Sci. 18, 109 (1978)
F. Bentiss, C. Jama, B. Mernari, H.E. Attari, L.E. Kadi, M. Lebrini, M. Traisnel, M. Lagrenée, Corrosion control of mild steel using 3,5-bis(4-methoxyphenyl)-4-amino-1,2,4-triazole in normal hydrochloric acid medium. Corros. Sci. 51, 1628 (2009)
S. Muralidharan, M.A. Quraishi, S.K.V. Iyer, The effect of molecular structure on hydrogen permeation and the corrosion inhibition of mild steel in acidic solutions. Corros. Sci. 37, 1739 (1995)
A.K. Singh, M.A. Quraishi, Effect of 2,2′ benzothiazolyl disulfide on the corrosion of mild steel in acid media. Corros. Sci. 51, 2752 (2009)
D. Wang, S. Li, Y. Ying, M. Wang, H. Xiao, Z. Chen, Theoretical and experimental studies of structure and inhibition efficiency of imidazoline derivatives. Corros. Sci. 41, 1911 (1999)
G. Bereket, C. Ogretir, C. Ozsahin, Quantum chemical studies on the inhibition efficiencies of some piperazine derivatives for the corrosion of steel in acidic medium. J. Mol. Struct. (THEOCHEM) 663, 39 (2003)
R.M. Issa, M.K. Awed, F.M. Atlam, Quantum chemical studies on the inhibition of corrosion of copper surface by substituted uracils. Appl. Surf. Sci. 255, 2433 (2008)
V.S. Sastri, J.R. Perumareddi, Molecular orbital theoretical studies of some organic corrosion inhibitors. Corrosion 53, 671 (1996)
T. Koopmans, Über die Zuordnung von Wellenfunktionen und Eigenwerten zu den Einzelnen Elektronen Eines Atoms. Physica 1, 104 (1934)
A. Szabo, N.S. Ostlund, Modern Quantum Chemistry; Introduction to Advanced Electronic Structure, Dover, 49 (1996)
C.T. Wang, S.H. Chen, H.Y. Ma, C.-S. Qi, Protection of copper corrosion by carbazole and N-vinylcarbazole self-assembled films in NaCl solution. J. Appl. Electrochem. 33, 179 (2003)
J. Fang, J. Li, Quantum chemistry study on the relationship between molecular structure and corrosion inhibition efficiency of amides. J. Mol. Struct. (Theochem) 593, 179 (2002)
P. Zhao, Q. Liang, Y. Li, Electrochemical, SEM/EDS and quantum chemical study of phthalocyanines as corrosion inhibitors for mild steel in 1 mol/l HCl. J. Appl. Surf. Sci. 252, 1596 (2005)
A.H. Mehaute, G. Grepy, Introduction to transfer and motion in fractal media: the geometry of kinetics. Solid State Ionic 9, 17 (1989)
L. Tang, X. Li, L. Li, G. Mu, G. Liu, Interfacial behavior of 4-(2-pyridylazo) resorcin between steel and hydrochloric acid. Surf. Coat. Technol. 201, 384 (2006)
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Singh, A.K., Khan, S., Singh, A. et al. Inhibitive effect of chloroquine towards corrosion of mild steel in hydrochloric acid solution. Res Chem Intermed 39, 1191–1208 (2013). https://doi.org/10.1007/s11164-012-0677-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-012-0677-8