Abstract
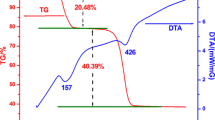
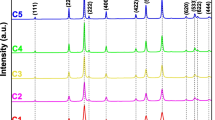
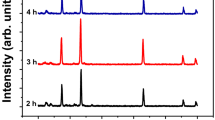
ZnO nanoparticles (NPs) with tunable morphologies were synthesized by a hybrid electrochemical–thermal method at different calcination temperatures without the use of any surfactant or template. The NPs were characterized by Fourier transform infrared (FT-IR) spectroscopy, X-ray diffraction, dynamic light scattering, thermogravimetry–differential thermal analysis, scanning electron microscope and N2 gas adsorption–desorption studies. The FT-IR spectra of ZnO NPs showed a band at 450 cm−1, a characteristic of ZnO, which remained fairly unchanged at calcination temperatures even above 300 °C, indicating complete conversion of the precursor to ZnO. The products were thermally stable above 300 °C. The ZnO NPs were present in a hexagonal wurtzite phase and the crystallinity of ZnO increased with an increasing calcination temperature. The ZnO NPs calcined at lower temperature were mesoporous in nature. The surface areas of ZnO NPs calcined at 300 and 400 °C were 51.10 and 40.60 m2 g−1, respectively, which are significantly larger than commercial ZnO nanopowder. Surface diffusion has been found to be the key mechanism of sintering during heating from 300 to 700 °C with the activation energy of sintering as 8.33 kJ mol−1. The photocatalytic activity of ZnO NPs calcined at different temperatures evaluated by photocatalytic degradation of methylene blue under sunlight showed strong dependence on the surface area of ZnO NPs. The ZnO NPs with high surface area showed enhanced photocatalytic activity.










Similar content being viewed by others
References
A.K. Radzimska, T. Jesionowski, Materials 7, 2833 (2014)
Z.L. Wang, J. Phys. Condens. Matter 16, 829 (2004)
C. Lizama, J. Freer, J. Baeza, H.D. Mansilla, Catal. Today 76, 235 (2002)
K. Qingshan, G. Chunxiang, S. Yuling, W. Haijuan, J. Quan, X. Yanzhi, Rare Met. 30, 213 (2011)
M. Rajamathi, S. Thimmaiah, P.E.D. Morgan, P. Seshadri, J. Mater. Chem. 11, 2489 (2001)
K. Zhu, B. Yue, W. Zhou, H. He, Chem. Commun. 1, 98 (2001)
Y.Q. Wang, C.M. Yang, W. Schmidt, B. Spliethoff, E. Bill, F. Schuth, Adv. Mater. 17, 53 (2005)
J. Sarkar, V.T. John, J. He, C. Brooks, D. Gandhi, A. Nunes, G. Ramanath, A. Bose, Chem. Mater. 20, 5301 (2008)
J.S. Beck, J.C. Vartuli, W.J. Roth, M.E. Leonowicz, C.T. Kresge, K.D. Schmitt, C.T.W. Chu, D.H. Olson, E.W. Sheppard, S.B. McCullen, J.B. Higgins, J.L. Schlenker, J. Am. Chem. Soc. 114, 10834 (1992)
C. Suwanchawalit, S. Wongnawa, J. Nanopart. Res. 12, 2895 (2010)
Z. Niu, Y. Li, Chem. Mater. 26, 72 (2014)
S.K. Pardeshi, A.B. Patil, J. Mol. Catal. A Chem. 308, 32 (2009)
C.W. Kim, U. Pal, S. Park, Y.H. Kim, J. Kim, Y.S. Kang, RSC Adv. 2, 11969 (2012)
K.G. Chandrappa, T.V. Venkatesha, K. Vathsala, C. Shivakumara, J. Nanopart. Res. 12, 2667 (2010)
C.K. Govindappa, V.T. Venkatarangaiah, S.B.A. Hamid, Nano-Micro Lett. 5, 101 (2013)
R. Wahab, S.G. Ansari, Y.S. Kim, M.A. Dar, H.S. Shin, J. All. Comp. 461, 66 (2008)
S. Syeeda, M.S. Miran, M.Y.A. Mollah, M.M. Rahman, J. Bangladesh Chem. Soc. 21, 129 (2008)
P. Ahmed, M.S. Miran, M.A.B.H. Susan, M.Y.A. Mollah, J. Bangladesh Chem. Soc. 26, 60 (2013)
S.S. Satter, M. Hoque, M.M. Rahman, M.Y.A. Mollah, M.A.B.H. Susan, RSC Adv. 4, 20612 (2014)
A. Escobedo-Morales, D. Téllez-Flores, M.L.R. Peralta, J. Garcia-Serrano, A.M. Herrera-González, E. Rubio-Rosas, E. Sánchez-Mora, O.O. Xometl, Mater. Chem. Phys. 151, 282 (2015)
F. Hassan, M.S. Miran, H.A. Simol, M.A.B.H. Susan, M.Y.A. Mollah, Bangladesh J. Sci. Ind. Res. 50, 21 (2015)
S. Zhaorigetu, Y. Hongxia. Garidi, Front. Chem. China 3, 277 (2006)
V. Vágvölgyi, M. Hales, W. Martens, J. Kristóf, E. Horváth, R.L. Frost, J. Therm. Anal. Calorim. 92, 911 (2008)
Z. Xianxi, W. Xiaojuan, Z. Guanjie, J. Jianzhuang, Chin. J. Inorg. Chem. 18, 1038–1040 (2002)
Y. Wang, S. Zhang, S. Bi, G. Luo, Powder Technol. 202, 130 (2010)
M.H. Rashid, M. Raula, R.R. Bhattacharjee, T.K. Mandal, J. Colloid Interface Sci. 339, 249 (2009)
Y. Gupta, A. Mansingh, J. Appl. Phys. 80, 1063 (1996)
V.D. Mote, Y. Purushotham, B.N. Dole, J. Theor. Appl. Phys. 6, 1 (2012)
J. Cheng, K.M. Poduska, Nanomaterials 3, 317 (2013)
C. Liewhiran, S. Phanichphant, J. Microsc. Soc. Thail. 20, 49 (2006)
D. Dollimore, P. Spooner, Trans. Faraday Soc. 67, 2750 (1971)
J.T. Cahill, J.N. Ruppert, B. Wallis, Y. Liu, O.A. Graeve, Langmuir 30, 5585 (2014)
O.A. Graeve, A. Madadi, R. Kanakala, K. Sinha, Metall. Mater. Trans. A 41, 2691 (2010)
D. Louer, R. Vargas, J.P. Auffredic, J. Am. Ceram. Soc. 67, 136 (1984)
K.S.W. Sing, D.H. Everett, R.A.W. Haul, L. Moscou, R.A. Pierotti, J. Rouquerol, T. Siemieniewska, Pure Appl. Chem. 57, 603 (1985)
G. Leofantia, M. Padovanb, G. Tozzolac, B. Venturelli, Catal. Today 41, 207 (1998)
X. Wang, Y. Zhang, C. Hao, F. Feng, H. Yin, N. Si, Ind. Eng. Chem. Res. 53, 6585 (2014)
J. Rouquerol, D. Avnir, C.W. Fairbridge, D.H. Everett, J.H. Haynes, N. Pernicone, J.D.F. Ramsay, K.S.W. Sing, K.K. Unger, Pure Appl. Chem. 66, 1739 (1994)
J.P. Auffrédic, A. Boultif, J.I. Langford, D. Louër, J. Am. Ceram. Soc. 78, 323 (1995)
P.D.L. Mercera, J.G.V. Ommen, E.B.M. Doesburg, A.J. Burggraaf, J.R.H. Ross, Appl. Catal. 57, 127 (1990)
F. Li, L. Chen, Z. Chen, J. Xu, J. Zhu, X. Xin, Mater. Chem. Phys. 73, 335 (2002)
W.H. Lai, L.G. Teoh, Y.H. Su, J. Shieh, M.H. Hon, J. Am. Ceram. Soc. 90, 4073 (2007)
O.J. Whittemore, Powder Technol. 29, 167 (1981)
A.S. Edelstein, R.C. Cammarata, Nanomaterials Synthesis, Properties and Applications, 1st edn. (IOP Publishing Ltd, UK, 1996)
R. Pampuch, K. Haberko, Agglomerates in ceramic, micropowders and their behaviour on cold pressing and sintering, in Ceramic Powders, ed. by P. Vincenzini (Elsevier, Amsterdam, 1983), pp. 623–634
J. Haber, Pure Appl. Chem. 63, 1227 (1991)
B.D. Zdravkov, J.J. Čermák, M. Šefara, J. Janků, Cent. Eur. J. Chem. 5, 385 (2007)
L. Zhonghao, A. Gebner, J.P. Richters, J. Kalden, T. Voss, C. K€ubel, A. Tauber, Adv. Mater. 20, 1279 (2008)
S. Kaluza, M.K. Schröter, R.N. d’Alnoncourt, T. Reinecke, M. Muhler, Adv. Funct. Mater. 18, 3670 (2008)
S. Polarz, A.V. Orlov, F. Schüth, A.H. Lu, Chem. Eur. J. 13, 592 (2007)
G. Xiong, L. Luo, C. Li, X. Yang, Energy Fuels 23, 1342 (2009)
D. Nicholson, Trans. Faraday Soc. 61, 990 (1965)
G.F. Hüttig, Kolloid Z. 98, 263 (1942)
S.J. Gregg, J. Chem. Soc. 3940 (1953). doi:10.1039/JR9530003940
G.A. El-Shobaky, N.S. Petro, Surf. Technol. 13, 197 (1981)
G.A. El-Shobaky, I.F. Hewaidy, T. El-Nabarawy, Surf. Technol. 12, 309 (1981)
H.G. El-Shobakya, M. Mokhtarb, G.A. El-Shobaky, Appl. Catal. A Gen. 180, 335 (1999)
N.A.M. Deraz, Colloid Surface. A 190, 251 (2001)
N. Obradovic, S. Stevanovic, V. Zeljkovic, M.M. Ristic, Powder Metall. Met. Ceram. 48, 182 (2009)
O. Mekasuwandumrong, P. Pawinrat, P. Praserthdam, J. Panpranot, Chem. Eng. J. 164, 77 (2010)
N.M. Flores, U. Pal, R. Galeazzi, A. Sandovalb, RSC Adv. 4, 41099 (2014)
H. Feng, M.H. Zhang, L.E. Yu, Appl. Catal. A Gen. 1413, 238 (2012)
J.Z. Kong, A.D. Li, X.Y. Li, H.F. Zhai, W.Q. Zhang, Y.P. Gong, H. Li, D. Wu, J. Solid State Chem. 183, 1359 (2010)
R.M. Mohamed, E.S. Baeissa, I.A.M. Khalid, M.A. Al-Rayyani, Appl Nano Sci. 3, 57 (2013)
M.A. Ali, M.R. Idris, M.E. Quayum, J. Nanostruct. Chem. 3, 36 (2013)
Acknowledgments
The authors acknowledge financial support for a sub-project (CP-231) from the Higher Education Quality Enhancement Project of the University Grants Commission of Bangladesh financed by the World Bank and the Government of Bangladesh. Technical support from the Centre for Advanced Research in Sciences (CARS) of the University of Dhaka is also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shohel, M., Miran, M.S., Susan, M.A.B.H. et al. Calcination temperature-dependent morphology of photocatalytic ZnO nanoparticles prepared by an electrochemical–thermal method. Res Chem Intermed 42, 5281–5297 (2016). https://doi.org/10.1007/s11164-015-2358-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-015-2358-x