Abstract
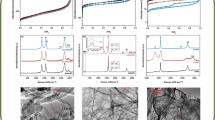
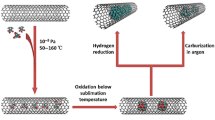
Water solubilization of carbon nanoparticles (nanocarbons), single-walled nanotubes (SWCNTs), nano-onions (NOs) and nanodiamonds (NDs) has been achieved through their covalent functionalization by fluorination and subsequent derivatization with sucrose. The covalent bonding of sucrose to the surface of the fluorinated nanocarbons was attained by a one-step fluorine substitution reaction with sucrose-derived lithium monosucrate under sonication in DMF at room temperature. This chemical process provides a simple, inexpensive, and easily scalable method for hydrophilic chemical modification of SWCNT, NO, and ND surfaces to produce sucrose-functionalized nanocarbons that become soluble in water, DMF, ethanol, and other polar solvents. The sucrose-functionalized nanocarbon particles are expected to be biocompatible due to the abundance of hydroxyl groups available for hydrogen bonding and further chemical modification. Relevant examples have been given.
Similar content being viewed by others
References
E. G. Rakov, Russ. Chem. Revs, 2001, 70, 827; (b) A. Hirsch, Angew. Chem., Int. Ed., 2002, 41, 1853; (c) A. Hirsh, O. Vostrowsky, Top. Curr. Chem., 2005, 245, 193.
S. Banerjee, T. Hemraj-Benny, S. S. Wong, Adv. Mater., 2005, 17, 17.
Y. Show, M. A. Witek, P. Sonthalia, G. M. Swain, Chem. Mater., 2003, 15, 879.
E. Wilks, J. Wilks, Properties and Applications of Diamond, Butterworth, Oxford, England, 1997.
M. Choi, I. S. Altman, Y. J. Kim, P. V. Pikhitsa, S. Lee, G. S. Park, T. Jeong, J. B. Yoo, Adv. Mater., 2004, 16, 1721.
J. C. Francis, Solid Lubricants and Self-lubricating Solids, Academic Press, New York, 1972.
Y. Liu, R. L. Vander Wal, V. N. Khabashesku, Chem. Mater., 2007, 19, 778.
A. Bianco, K. Kostarelos, C. D. Partidos, M. Prato, Chem. Commun., 2005, 571.
A. S. Rettenbacher, B. Elliott, J. S. Hudson, A. Amirkhanian, L. Echegoyen, Chem. Eur. J., 2005, 12, 376.
A. Krüger, Y. Liang, G. Jarre, J. J. Stegk, Mater. Chem., 2006, 16, 2322.
J. J. Stephenson, J. L. Hudson, A. D. Leonard, B. K. Price, J. M. Tour, Chem. Mater., 2007, 19, 3491.
L. Feng, J. M. Beach, P. K. Rai, W. Guo, R. H. Hauge, M. Pasquali, R. E. Smally, W. E. Billups, Chem. Mater., 2006, 18, 1520.
V. N. Khabashesku, W. E. Billups, J. L. Margrave, Acc. Chem. Res., 2002, 35, 1087; (b) Y. S. Lee, T. H. Cho, B. K. Lee, J. S. Rho, K. H. An, Y. H. Lee, J. Fluorine Chem., 2003, 120, 99; (c) A. V. Krestinin, A. P. Kharitonov, Yu. M. Shul’ga, O. M. Zhigalina, E. I. Knerel’man, M. Dubois, M. M. Brzhezinskaya, A. S. Vinogradov, A. B. Preobrazhenskii, G. I. Zvereva, M. B. Kislov, V. M. Martynenko, I. I. Korobov, G. I. Davydova, V. G. Zhigalina, N. A. Kiselev, Nanotech_ nologies in Russia, 2009, 4, 115.
M. Burghard, Surface Sci. Reports, 2005, 58, 1; (b) V. N. Khabashesku, M. X. Pulikkathara, Mendeleev Commun., 2006, 61.
V. N. Khabashesku, Fluorination of Carbon Nanotubes, in Chemistry of Carbon Nanotubes, Eds V. A. Basiuk, E. A. Basiuk, Stevenson Ranch, CA: ASP Publishers, 2007, Vol. 1, 522; (b) V. N. Khabashesku, O. V. Kuznetsov, M. X. Pulikkathara, Carbon Nanotubes: Fluorinated Derivatives in Nanomaterials: Inorganic and Bioinorganic Perspectives, Eds C. M. Lukehart, R. A. Scott, John Wiley and Sons, Ltd, Chichester, 2009, 856 pp.
Y. Liu, Z. Gu, J. L. Margrave, V. N. Khabashesku, Chem. Mater., 2004, 16, 3924.
V. N. Khabashesku, H. Peng, J. L. Margrave, Nanotube- Amino acids and Methods for Preparing Same (WO/2005/ 070828), Internat. Pat. Application No.: PCT/US2005/ 001310, Internat. Filing Date: 18.01.200, Priority Data: 60/537,982 21.01.2004 US.
M. X. Pulikkatara, V. N. Khabashesku, Izv. Akad. Nauk. Ser. Khim., 2008, 1035 [Russ. Chem. Bull., Int. Ed., 2008, 57, 1054].
M. X. Pulikkathara, O. V. Kuznetsov, V. N. Khabashesku, Chem. Mater., 2008, 20, 2685.
L. Zhang, V. U. Kiny, H. Peng, R. F. M. Lobo, J. L. Margrave, V. N. Khabashesku, Chem. Mater., 2004, 16, 2055.
S. J. V. Frankland, A. Caglar, D. W. Brenner, M. J. Griebel, J. Phys. Chem. B, 2002, 106, 3046.
J. N. Coleman, U. Khan, Y. K. Gun’ko, Adv. Mater., 2006, 18, 689.
P. Nikolaev, M. J. Bronikowski, R. K. Bradley, F. Rohmund, D. T. Colbert, K. A. Smith, R. E. Smalley, Chem. Phys. Lett., 1999, 313, 91; (b) R. L. Vander Wal, A. J. Tomasek, K. W. Street, D. R. Hull, W. K. Thompson, Appl. Spectros?., 2004, 58, 230.
C. A. Browne, A Handbook of Sugar Analysis, John Wiley and Sons, New York; Chapman and Hall, London, 1912, p. 676.
M. Manley-Harris, W. Moody, G. N. Richards, Austral. J. Chem., 1980, 33, 1041.
J. Fitremann, Y. Queneau, J.-P. Maitre, A. Bouchu, Tetrahedron Lett., 2007, 48, 4111.
P. E. Shaw, J. H. Tatum, R. E. Berry, J. Agr. Food. Chem., 1969, 17, 907.
P. L. Polavarapu, S. R. Chatterjee, D. F. Michalska, Carbohydrate Res., 1985, 137, 253.
M. V. Korolevich, R. G. Zhbankov, V. V. Sivchik, J. Mol. Struct., 1990, 220, 301.
J. W. Ager III, D. K. Veirs, G. M. Rosenblatt, Phys. Rev. B, 1991, 43, 6491.
A. C. Ferrari, J. Robertson, Phys. Rev. B, 2000, 61, 14095.
V. A. Davydov, A. V. Rakhmanina, S. Rols, V. Agafonov, M. X. Pulikkathara, R. L. Vander Wal, J. Phys. Chem. C, 2007, 111, 12918.
S. Wang, R. Liang, B. Wang, C. Zhang, Nanotechnology, 2008, 19, 085710.
N. P. G. Roeges, Guide to the Complete Interpretation of Infrared Spectra of Organic Structures, Jonn Wiley and Sons Publ., Chichester-New York-Brisbane-Toronto- Singapore, 1994.
Author information
Authors and Affiliations
Corresponding author
Additional information
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 8, pp. 1462-1472, August, 2010.
Rights and permissions
About this article
Cite this article
Kuznetsov, O.V., Pulikkathara, M.X., Lobo, R.F.M. et al. Solubilization of carbon nanoparticles, nanotubes, nano-onions, and nanodiamonds through covalent functionalization with sucrose. Russ Chem Bull 59, 1495–1505 (2010). https://doi.org/10.1007/s11172-010-0269-y
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-010-0269-y