Abstract
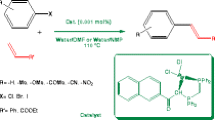
A novel pathway for cross-coupling reaction of terminal alkenes with aryl halides (Heck cross-coupling) has been described using a new phosphine-free poly(N-vinyl carbazole) anchored palladium(II) complex as catalyst in aerobic conditions. The catalyst was found to be highly active for the couplings of a variety of substituted and non-substituted aryl halides with terminal alkenes and to smoothly afford the corresponding desired products in good to excellent yields. The catalyst can be reused at least six times without noticeable decrease in catalytic activity.




Similar content being viewed by others
References
Heck RF, Nolley JP (1972) J Org Chem 37:2320–2322
Plevyak JE, Heck RF (1978) J Org Chem 43:2454–2456
Nicolaou KC, Bulger PG, Sarlah D (2005) Angew Chem Int Ed 44:4442–4489
Cabri AW, Candiani I (1995) Acc Chem Res 28:2–7
Herrman WA, Bromer C, Öfele K, Reisinger C, Riermeier T, Beller M, Fischer H (1995) Angew Chem 107:1989
Burke TR, Liu D-G, Gao Y (2000) J Org Chem 65:6288–6291
Haberli A, Leumann CJ (2001) Org Lett 3:489–492
Link JT (1998) Overman LE metal-catalyzed cross-coupling reactions. In: Diederich F, Stang PJ (eds) Wiley-VCH Verlag, Weinheim, p 231
Bader RR, Baumeister P, Blaser HU (1996) Chimia 50:99
Wu TC (1996) US Patent, 5536870
Eisenstadt A (1998) Palladium in catalysis of organic reactions. In: Herkes FE, Dekker M (eds) New York
Zhao F, Bhanage BM, Shirai M, Arai M (1999) J Mol Catal A Chem 142:383–388
Brase S, De Meijere A (1998) Metal-catalyzed cross-coupling reactions. Wiley-VCH, Weinheim, p 99
Barder TE, Walker SD, Martinelli JR, Buchwald SL (2005) J Am Chem Soc 127:4685–4696
Akba O, Durap F, Aydemir M, Baysal A, Gümgüma B, Özkar S (2009) J Organometal Chem 694:731–736
Surawatanawong P, Fan Y, Hall MB (2008) J Organometal Chem 693:1552–1563
Wang YD, Dutia M, Floyd MB, Prashad AS, Berger D, Lin M (2009) Tetrahedron 65:57–61
Wang PW, Fox MA (1994) J Org Chem 59:5358–5364
Tulloch AAD, Danopoulos AA, Tooze RP, Cafferkey SM, Kleinhenz S, Hursthouse MB (2000) Chem Commun 1247–1248
Shang Y, Wu J, Fan C, Hu J, Lu B (2008) J Organometal Chem 693:2963–2966
Cwik A, Hell Z, Figueras F (2006) Adv Synth Catal 348:523–530
Peris E, Loch JA, Mata J, Crabtree RH (2001) Chem Commun 201–202
Selvakumar K, Zapf A, Beller M (2002) Org Lett 4:3031–3033
Jung IG, Son SU, Park KH, Chung K-C, Lee JW, Chung YK (2003) Organometallics 22:4715–4720
Dıez-Barra E, Guerra J, Hornillos V, Merino S, Tejeda J (2003) Organometallics 22:4610–4612
Consorti CS, Zanini ML, Leal S, Ebeling G, Dupont J (2003) Org Lett 5:983–986
Najera C, Gil-Molto J, Karlstrom S, Falvello LR (2003) Org Lett 5:1451–1454
Masllorens J, Moreno-Manas M, Pla-Quintana A, Roglans A (2003) Org Lett 5:1559–1561
Park SB, Alper H (2003) Org Lett 5:3209–3212
Mazet C, Gade LH (2003) Eur J Inorg Chem 1161–1168
Gladysz JA (2000) Recoverable catalyst, reagents perspective, prospective. Chem Rev 102:3215–3216
Sherrington DC (1998) Chem Commun 2275–2286
Polshettiwar V, Molnar A (2007) Tetrahedron 63:6949–6976
Polshettiwar V, Varma RS (2008) Tetrahedron 64:4637–4643
Song D, Yi WB (2008) J Mol Catal A Chem 280:20–23
Budarin VL, Clark JH, Luque R, Macquarrie DJ, White RJ (2008) Green Chem 10:382–387
Chandrasekhar S, Narsihmulu C, Sultana SS, Reddy NR (2002) Org Lett 4:4399–4401
Gniewek A, Trzeciak AM, Ziółkowski JJ, Kepinski L, Wrzyszcz J, Tylus W (2005) J Catal 229:332–343
Beletskaya IP, Kashin AN, Litvinov AE, Tyurin VS, Valetsky PM, van Koten G (2006) Organometallics 25:154–158
Ribière P, Declerck V, Nédellec Y, Yadav-Bhatnagar N, Martinez J, Lamaty F (2006) Tetrahedron 62:10456–10466
Dahan A, Portnoy M (2003) Org Lett 5(8):1197–1200
Meier MAR, Filali M, Gohy J-F, Schubert US (2006) J Mater Chem 16:3001–3006
Steel PG, Teasdale CWT (2004) Tetrahedron Lett 45:8977–8980
King RB, Sweet EM (1979) J Org Chem 44:385–391
Kharasch MS, Seyler RC, Mayo FR (1983) J Am Chem Soc 60:822–887
Onue H, Moritani I (1972) J Organomet Chem 43:431–436
Durig JR, Layton R, Sink DW, Mitchell BR (1965) Spectrochim Acta 21:1367–1378
Santra PK, Sagar P (2003) J Mol Catal A Chem 197:37–50
Lever ABP (1968) Inorganic electronic spectroscopy, 1st ed. Elsevier, Amsterdam
Molnar A, Papp A (2006) Synlett 3130–3134
Prockl SS, Kleist W, Kohler K (2005) Tetrahedron 61:9855–9859
Polshettiwar V, Hesemann P, Moreau JJE (2007) Tetrahedron Lett 48:5363–5366
Tsai FY, Wu CL, Mou CY, Chao MC, Linc HP, Liua ST (2004) Tetrahedron Lett 45:7503–7506
Cai M, Huanga Y, Zhaob H, Songa C (2004) Reac Func Polymers 59:81–86
Chen T, Gaob J, Shi M (2006) Tetrahedron 62:6289–6294
Nandurkar NS, Bhanage BM (2008) Tetrahedron 64:3655–3660
Acknowledgments
We acknowledge the Department of Science and Technology (DST), Council of Scientific and Industrial Research (CSIR) and the University Grant Commission (UGC), New Delhi, India for funding. One of the authors, K.T., is thankful to the University Grants Commission (Eastern Region), India, for financial support. We also thank the DST and UGC, New Delhi, India for providing instrumental support under the FIST and SAP program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Islam, M., Mondal, P., Roy, A.S. et al. Use of a recyclable poly(N-vinyl carbazole) palladium(II) complex catalyst: Heck cross-coupling reaction under phosphine-free and aerobic conditions. Transition Met Chem 35, 491–499 (2010). https://doi.org/10.1007/s11243-010-9354-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11243-010-9354-1