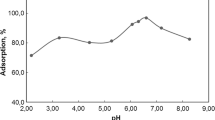
Abstract
The processing of natural resources in marine and freshwater ecosystems, directly operated by industries related to maritime sector, contributes annually to several million tons of waste. The reuse and economic recovery of this waste would be very desirable and profitable, either economically or environmentally. In this work, the remediation of hazardous divalent metal ions from aqueous solutions using biological apatites derived from marine residues was addressed. The biological apatite (calcium phosphate particles) was produced by heat treatment of fish bones. Experimental sorption studies of kinetics and equilibrium of the metal ions as well as an evaluation of competitive sorption behavior for lead immobilization were carried out in batch operation mode. The efficiency and mechanisms of lead sorption on two different particle sizes of calcium phosphate from aqueous solution were investigated. The results showed a high adsorption capacity of the biosorbent for Pb2+ (above 370 mg/gads.), in opposition to Cd2+ and Zn2+. For the case of low initial concentrations of the metal ion, reducing the biosorbent particles size increases the sorption rate. It was possible to verify that lead immobilization proceeds with a rapid surface complexation of the lead on the sorption sites before partial dissolution of calcium phosphate and formation of pyromorphite-like compounds. By the selectivity experiments, performed using binary systems—Pb/Cd and Pb/Zn—it was evidenced a competitive process between the divalent ions, which leads to a considerable decrease on the adsorption capacity of the adsorbent material for all the metals.






Similar content being viewed by others
References
Admassu, W., & Breese, T. (1999). Feasibility of using natural fishbone apatite as a substitute for hydroxyapatite in remediating aqueous heavy metals. Journal of Hazardous Materials, 69(2), 187–196.
Bailliez, S., Nzihou, A., Bèche, E., & Flamant, G. (2004). Removal of lead (Pb) by hydroxyapatite sorbent. Process Safety and Environmental Protection, 82(2), 175–180.
Boutinguiza, M., Pou, J., Comesaña, R., Lusquiños, F., de Carlos, A., & León, B. (2011). Biological hydroxyapatite obtained from fish bones. Materials Science and Engineering C (in press).
Boutinguiza, M., Pou, J., Lusquiñosa, F., Comesaña, R., & Riveiro, A. (2010). Laser-assisted production of tricalcium phosphate nanoparticles from biological and synthetic hydroxyapatite in aqueous medium. Applied Surface Science. doi:10.1016/j.apsusc.2010.12.004.
Cao, X., Ma, L. Q., Rhue, D. R., & Appel, C. S. (2004). Mechanisms of lead, copper, and zinc retention by phosphate rock. Environmental Pollution, 131(3), 435–444.
Chen, X., Wright, J. V., Conca, J. L., & Peurrung, L. M. (1997a). Effects of pH on heavy metal sorption on mineral apatite. Environmental Science and Technology, 31(3), 624–631.
Chen, X., Wright, J., Conca, J., & Peurrung, L. (1997b). Evaluation of heavy metal remediation using mineral apatite. Water, Air, and Soil Pollution, 98(1), 57–78.
Cheung, C. W., Chan, C. K., Porter, J. F., & McKay, G. (2001). Combined diffusion model for the sorption of cadmium, copper, and zinc ions onto bone char. Environmental Science & Technology, 35(7), 1511–1522.
Corami, A., Mignardi, S., & Ferrini, V. (2007). Copper and zinc decontamination from single- and binary-metal solutions using hydroxyapatite. Journal of Hazardous Materials, 146(1–2), 164–170.
Corami, A., Mignardi, S., & Ferrini, V. (2008). Cadmium removal from single- and multi-metal (Cd + Pb + Zn + Cu) solutions by sorption on hydroxyapatite. Journal of Colloid and Interface Science, 317(2), 402–408.
da Rocha, N. C. C., de Campos, R. C., Rossi, A. M., Moreira, E. L., Barbosa, A. D. F., & Moure, G. T. (2002). Cadmium uptake by hydroxyapatite synthesized in different conditions and submitted to thermal treatment. Environmental Science and Technology, 36(7), 1630–1635.
Davis, T. A., Volesky, B., & Mucci, A. (2003). A review of the biochemistry of heavy metal biosorption by brown algae. Water Research, 37(18), 4311–4330.
Deydier, E., Guilet, R., & Sharrock, P. (2003). Beneficial use of meat and bone meal combustion residue: "an efficient low cost material to remove lead from aqueous effluent". Journal of Hazardous Materials, 101(1), 55–64.
Dybowska, A., Manning, D. A. C., Collins, M. J., Wess, T., Woodgate, S., & Valsami-Jones, E. (2009). An evaluation of the reactivity of synthetic and natural apatites in the presence of aqueous metals. Science of the Total Environment, 407(8), 2953–2965.
Elliott, J. C. (1994). Structure and chemistry of the apatites and other calcium orthophosphates (studies in inorganic chemistry) (1st ed.). Amsterdam: Elsevier.
Fernane, F., Mecherri, M. O., Sharrock, P., Hadioui, M., Lounici, H., & Fedoroff, M. (2008). Sorption of cadmium and copper ions on natural and synthetic hydroxylapatite particles. Materials Characterization, 59(5), 554–559.
Hankermeyer, C. R., Ohashi, K. L., Delaney, D. C., Ross, J., & Constantz, B. R. (2002). Dissolution rates of carbonated hydroxyapatite in hydrochloric acid. Biomaterials, 23(3), 743–750.
Jeanjean, J., McGrellis, S., Rouchaud, J. C., Fedoroff, M., Rondeau, A., Perocheau, S., et al. (1996). A crystallographic study of the sorption of cadmium on calcium hydroxyapatites: incidence of cationic vacancies. Journal of Solid State Chemistry, 126(2), 195–201.
Kaludjerovic-Radoicic, T., & Raicevic, S. (2010). Aqueous Pb sorption by synthetic and natural apatite: kinetics, equilibrium and thermodynamic studies. Chemical Engineering Journal, 160(2), 503–510.
Kovaleva, E. S., Shabanov, M. P., Putlyaev, V. I., Tretyakov, Y. D., Ivanov, V. K., & Silkin, N. I. (2009). Bioresorbable carbonated hydroxyapatite Ca10-xNax(PO4)(6-x)(CO3)(x)(OH)(2) powders for bioactive materials preparation. Central European Journal of Chemistry, 7(2), 168–174.
LeGeroes, R. Z., & LeGeroes, J. P. (1984). Phosphate minerals in human tissues. In J. O. Nariagu (Ed.), Phosphate minerals (pp. 351–385). Berlin: Springer.
Liao, D., Zheng, W., Li, X., Yang, Q., Yue, X., Guo, L., et al. (2010). Removal of lead(II) from aqueous solutions using carbonate hydroxyapatite extracted from eggshell waste. Journal of Hazardous Materials, 177(1–3), 126–130.
Lower, S. K., Maurice, P. A., & Traina, S. J. (1998). Simultaneous dissolution of hydroxylapatite and precipitation of hydroxypyromorphite: direct evidence of homogeneous nucleation. Geochimica Et Cosmochimica Acta, 62(10), 1773–1780.
Ma, L. Q. Y. (1996). Factors influencing the effectiveness and stability of aqueous lead immobilization by hydroxyapatite. Journal of Environmental Quality, 25(6), 1420–1429.
Ma, Q. Y., Traina, S. J., Logan, T. J., & Ryan, J. A. (1993). In situ lead immobilization by apatite. Environmental Science and Technology, 27(9), 1803–1810.
Ma, Q. Y., Traina, S. J., Logan, T. J., & Ryan, J. A. (1994). Effects of aqueous Al, Cd, Cu, Fe(II), Ni, and Zn on Pb immobilization by hydroxyapatite. Environmental Science and Technology, 28(7), 1219–1228.
Mandjiny, S., Zouboulis, A. I., & Matis, K. A. (1995). Removal of cadmium from dilute-solutions by hydroxyapatite .1. Sorption studies. Separation Science and Technology, 30(15), 2963–2978.
Marchat, D., Bernache-Assollant, D., & Champion, E. (2007). Cadmium fixation by synthetic hydroxyapatite in aqueous solution—thermal behaviour. Journal of Hazardous Materials, 139(3), 453–460.
Mavropoulos, E., Rocha, N. C. C., Moreira, J. C., Rossi, A. M., & Soares, G. A. (2004). Characterization of phase evolution during lead immobilization by synthetic hydroxyapatite. Materials Characterization, 53(1), 71–78.
Mavropoulos, E., Rossi, A. M., Costa, A. M., Perez, C. A. C., Moreira, J. C., & Saldanha, M. (2002). Studies on the mechanisms of lead immobilization by hydroxyapatite. Environmental Science and Technology, 36(7), 1625–1629.
Middelburg, J. J., & Comans, R. N. J. (1991). Sorption of cadmium on hydroxyapatite. Chemical Geology, 90(1–2), 45–53.
Monteil-Rivera, F., & Fedoroff, M. (2002). Sorption of inorganic species on apatites from aqueous solutions. In Encyclopedia of surface and colloid science (pp. 1–26). New York: Marcel Dekker.
Mouflih, M., Aklil, A., Jahroud, N., Gourai, M., & Sebti, S. (2006). Removal of lead from aqueous solutions by natural phosphate. Hydrometallurgy, 81(3–4), 219–225.
Nelson, D. G. A. (1981). The influence of carbonate on the atomic-structure and reactivity of hydroxyapatite. Journal of Dental Research, 60, 1621–1629.
Peld, M., Tonsuaadu, K., & Bender, V. (2004). Natural and synthetic apatites as sorbents for Cd2+ and Cr3+ ions from aqueous solutions. Proceedings of the Estonian Academy of Sciences. Chemistry, 53(2), 75–90.
Prasad, M., Saxena, S., Amritphale, S. S., & Chandra, N. (2000). Kinetics and isotherms for aqueous lead adsorption by natural minerals. Industrial and Engineering Chemistry Research, 39(8), 3034–3037.
Sandrine, B., Ange, N., Didier, B.-A., Eric, C., & Patrick, S. (2007). Removal of aqueous lead ions by hydroxyapatites: equilibria and kinetic processes. Journal of Hazardous Materials, 139(3), 443–446.
Sery, A., Manceau, A., & Greaves, G. N. (1996). Chemical state of Cd in apatite phosphate ores as determined by EXAFS spectroscopy. American Mineralogist, 81(7–8), 864–873.
Singh, S. P., Ma, L. Q., & Harris, W. G. (2001). Heavy metal interactions with phosphatic clay. Journal of Environmental Quality, 30(6), 1961–1968.
Smiciklas, I., Onjia, A., & Raicevic, S. (2005). Experimental design approach in the synthesis of hydroxyapatite by neutralization method. Separation and Purification Technology, 44(2), 97–102.
Smiciklas, I., Onjia, A., Raicevic, S., Janackovic, Ð., & Mitric, M. (2008). Factors influencing the removal of divalent cations by hydroxyapatite. Journal of Hazardous Materials, 152(2), 876–884.
Sugiyama, S., Ichii, T., Fujii, M., Fujisawa, M., Kawashiro, K., & Hayashi, H. (2002). Facile preparation of lead hydroxyapatite through lead immobilization by calcium hydrogen phosphates in aqueous solutions. Inorganic Chemistry Communications, 5(8), 606–608.
Suzuki, T., Hatsushika, T., & Miyake, M. (1982). Synthetic hydroxyapatites as inorganic cation exchangers. Part 2. Journal of the Chemical Society-Faraday Transactions I, 78, 3605–3611.
Suzuki, Y., & Takeuchi, Y. (1994). Uptake of a few divalent heavy-metal ionic species by a fixed-bed of hydroxyapatite particles. Journal of Chemical Engineering of Japan, 27(5), 571–576.
Takeuchi, Y., Suzuki, T., & Arai, H. (1988). A study of equilibrium and mass-transfer in processes for removal of heavy-metal ions by hydroxyapatite. Journal of Chemical Engineering of Japan, 21(1), 98–100.
Tang, R. K., Henneman, Z. J., & Nancollas, G. H. (2003). Constant composition kinetics study of carbonated apatite dissolution. Journal of Crystal Growth, 249(3–4), 614–624.
Tang, R. K., Wang, L. J., & Nancollas, G. H. (2004). Size-effects in the dissolution of hydroxyapatite: an understanding of biological demineralization. Journal of Materials Chemistry, 14(14), 2341–2346.
Valsami-Jones, E., Ragnarsdottir, K. V., Putnis, A., Bosbach, D., Kemp, A. J., & Cressey, G. (1998). The dissolution of apatite in the presence of aqueous metal cations at pH 2–7. Chemical Geology, 151(1–4), 215–233.
Wu, L. M., Forsling, W., & Schindler, P. W. (1991). Surface complexation of calcium minerals in aqueous-solution. 1. Surface protonation at fluorapatite water interfaces. Journal of Colloid and Interface Science, 14(1), 178–185.
Xu, Y., Schwartz, F. W., & Traina, S. J. (1994). Sorption of Zn2+ and Cd2+ on hydroxyapatite surfaces. Environmental Science and Technology, 28(8), 1472–1480.
Zhang, Z., Li, M., Chen, W., Zhu, S., Liu, N., & Zhu, L. (2010). Immobilization of lead and cadmium from aqueous solution and contaminated sediment using nano-hydroxyapatite. Environmental Pollution, 158(2), 514–519.
Zhu, R., Yu, R., Yao, J., Mao, D., Xing, C., & Wang, D. (2008). Removal of Cd2+ from aqueous solutions by hydroxyapatite. Catalysis Today, 139(1–2), 94–99.
Acknowledgments
This work was supported by the project IBEROMARE—Multipolar Center for Valorization of Marine Resources and Wastes, approved by Operational Programme for Cross-Border Cooperation: Spain, Portugal 2007–2013 (POCTEP), with funding contribution through the European Regional Development Fund (ERDF cofinancing) and POCTEP.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
da Silva, E.A.B., Costa, C.A.E., Vilar, V.J.P. et al. Water Remediation Using Calcium Phosphate Derived From Marine Residues. Water Air Soil Pollut 223, 989–1003 (2012). https://doi.org/10.1007/s11270-011-0918-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-011-0918-2