Abstract
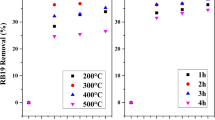
Utilization of industrial solid wastes for the treatment of wastewater from another industry could help environmental pollution abatement, in solving both solid waste disposal as well as liquid waste problems. Red mud (RM) is a waste product in the production of alumina and it poses serious pollution hazard. The present paper focuses on the possibility of utilization of RM as an adsorbent for removal of Remazol Brilliant Blue dye (RBB), a reactive dye from dye-contaminated water. Adsorption of RBB, from dye-contaminated water was studied by adsorption on powdered sulfuric acid-treated RM. The effect of initial dye concentration, contact time, initial pH, and adsorbent dosage were studied. Langmuir isotherm model has been found to represent the equilibrium data for RBB–RM adsorption system better than Freundlich model. The adsorption capacity of RM was found to be 27.8 mg dye/g of adsorbent at 40 °C. Thermodynamic analysis showed that adsorption of RBB on acid-treated RM is an endothermic reaction with ∆H 0 of 28.38 kJ/mol. The adsorption kinetics is represented by second-order kinetic model and the kinetic constant was estimated to be 0.0105 ± 0.005 g/mg min. Validity of intra-particle diffusion kinetic model suggested that among the mass transfer processes during the dye adsorption process, pore diffusion is the controlling step and not the film diffusion. The process can serve dual purposes of utilization of an industrial solid waste and the treatment of liquid waste.










Similar content being viewed by others
References
Agyei, N. M., Strydom, C. A., & Potgieter, J. H. (2000). An investigation of phosphate ion adsorption from aqueous solution by fly ash and slag. Cement and Concrete Research, 30(5), 823–826.
Aksu, Z., & Dönmez, D. (2003). A comparative study on the biosorption characteristics of some yeasts for Remazol Blue reactive dye. Chemosphere, 50, 1075–1083.
Aksu, Z., & Tezer, S. (2000). Equilibrium and kinetic modelling of biosorption of Remazol Black B by Rhizopusarrhizusin a batch system: effect of temperature. Process Biochemistry, 36(5), 431–439.
Annadurai, G., Juang, R., & Lee, D. (2002). Use of cellulosebased wastes for adsorption of dyes from aqueous solutions. Journal of Hazardous Materials, 92, 263–274.
Asfour, H. M., Fadali, O. A., Nassar, M. M., & EI-Geundi, M. S. (1985). Equilibrium studies on adsorption of basic dyes on hardwood. Journal of Chemical Technology and Biotechnology, 35A, 21–27.
Baup, S., Jaffre, C., Wolbert, D., & Laplanche, A. (2000). Adsorption of pesticides onto granulated activated carbon: determination of surface diffusivities using simple batch experiments. Adsorption, 6(3), 219–228.
Brown, M. A., & De Vito, S. C. (1993). Predicting azo dye toxicity. Critical Review in Environmental Science and Technology, 23, 249.
Danis, T. G., Albanis, T. A., Petrakis, D. E., & Pomonis, P. J. (1998). Removal of chlorinated phenols from aqueous solutions by adsorption on alumina pillared clays and mesoporous alumina aluminum phosphates. Water Research, 32, 295–302.
Eren, Z., & Acar, F. N. (2006). Adsorption of Reactive Black 5 from an aqueous solution:equilibrium and kinetic studies. Desalination, 194, 1–10.
Gong, R., Li, M., Yang, C., Sun, Y., & Chen, J. (2005). Removal of cationic dyes from aqueous solution by adsorption on peanut hull. Journal of Hazardous Materials, 121, 247–250.
Guclu, K., & Apak, R. (2000). Modeling of copper(II), cadmium (II) and lead (II) adsorption on red mud from metal–EDTA mixture solutions. Journal of Colloid and Interface Science, 228, 238–2525.
Gupta, V. K., Ali, I., Suhas, & Mohan, D. (2003). Equilibrium uptake and sorptiondynamics for the removal of a basic dye (basic red) using low-cost adsorbents. Journal of Colloid and Interface Science, 265, 257–264.
Gupta, V. K., Suhas, I. A., & Saini, V. K. (2004). Emoval of Rhodamine B, Fast Green and Methylene Blue from waste water using Red Mud, an aluminium industry waste. Industrial and Engineering Chemistry Research, 43, 1740–1747.
Hayrunnisa, N., & Ekrem, K. (2012). Removal of cobalt (II) ions from aqueous solution by using alternative adsorbent industrial red mud waste material. International Journal of Physical Sciences, 7(9), 1386–1394.
Ho, Y. S., & McKay, G. (1998). Kinetic models for the sorption of dye from aqueous solution by wood. Process Safety and Environmental Protection, 76, 183–191.
Ho, Y. S., & McKay, G. (1999). Competitive sorption of copper and nickel ions from aqueous solution using peat. Adsorption-Journal of the International Adsorption Society, 5(4), 409–417.
Igwe, J. C., & Abia, A. A. (2007). Adsorption kinetics and intraparticulate diffusivities for bioremediation of Co (II), Fe (II) and Cu (II) ions from waste water using modified and unmodified maize cob. International Journal of Physical Sciences, 2(5), 119–127.
Jain, R., & Sikarwar, S. (2006). Photocatalytic and adsorption studies on the removal of dye Congo red from wastewater. International Journal of Environmental Pollution, 27, 158–178.
Jain, A. K., Gupta, V. K., Bhatnagar, A., Jain, S., & Suhas. (2003). A comparative assessment of adsorbents prepared from industrial wastes for the removal of cationic dye. Journal of Indian Chemical Society, 80, 267–270.
Jain, A. K., Gupta, V. K., Bhatnagar, A., & Suhas. (2003a). A comparative study of adsorbents prepared from industrial wastes for removal of dyes. Separation Science and Technology, 38, 463–481.
Jain, A. K., Gupta, V. K., Bhatnagar, A., & Suhas. (2003b). Utilization of industrial waste products as adsorbents for the removal of dyes. Journal of Hazardous Materials, 101, 31–42.
Juang, R. S., Wu, F. C., & Tseng, R. L. (2002). Characterization and use of activated carbons prepared from bagasses for liquid-phase adsorption. Colloids Surfactants A, 201, 191–199.
Khattri, S. D., & Singh, M. K. (1999). Color removal from dye waste water using sugarcane dust as an adsorbent. Adsorption Science and Technology, 17, 269–282.
Li, Y., Liu, C., & Chiou, C. (2003). Decolorization of acid blue 9 dye wastewater using waste furnace slag. Bulletin of Environmental Contamination and Toxicology, 70, 1112–1120.
Liu, C.-J., Li, Y.-Z., Luan, Z.-K., Chen, Z.-Y., Zhang, Z.-G., & Jia, Z.-P. (2007). Adsorption removal of phosphate from aqueous solution by active red mud. Journal of Environmental Sciences, 19, 1166–1170.
Lopezgalindo, A., Viseras, C., & Cerezo, P. (2007). Compositional, technical and safety specifications of clays to be used as pharmaceutical and cosmetic products. Applied Clay Science, 36, 51–63.
McKay, G., & Poots, V. J. P. (1980). Kinetics and diffusion processes in colour removal from effluent using wood as an adsorbent. Journal of Chemical Technology and Biotechnology, 30, 279–292.
McKay, G., Porter, J. F., & Prasad, G. R. (1999). The removal of dye colours from aqueous solutions by adsorption on low-cost materials. Water, Air, and Soil Pollution, 114, 423–438.
Mishra, G., & Tripathy, M. (1993). A critical review of the treatment for decolorization of dye wastewater. Colourage, 40, 35–38.
Mohamed, M. M. (2004). Comparison of surfactant-modified mesoporous FSM-16 with activated carbon derived from rice husk. Journal of Colloid and Interface Science, 272, 28–34.
Mohan, D., Singh, K. P., Singh, G., & Kumar, K. (2000). Removal of dyes from wastewater using flyash, a low-cost adsorbent. Industrial and Engineering Chemistry Research, 41, 3688–3695.
Namasivayam, C., & Arasi, D. J. S. E. (1997). Removal of congo red from wastewater by adsorption onto waste red mud. Chemosphere, 34(2), 401–417.
Namasivayam, C., Yamuna, R., & Arasi, D. (2001). Removal of acid violet from wastewater by adsorption on waste red mud. Environmental Geology, 41, 269–273.
Namasivayam, C., Yamuna, R., & Arasi, D. (2002). Removal of Procion orange from waste water by adsorption on waste red mud. Separation Science and Technology, 37(10), 2421–2431.
Norouzi, S., Badii, K., & Doulati Ardejani, F. (2010). Activated bauxite waste as an adsorbent for removal of Acid Blue 92 from aqueous solutions. Water Science and Technology, 62, 2491–2500.
Ozcan, S., Tor, A., & Aydin, M. E. (2011). Removal of organochlorine pesticides from aqueous solution by using neutralized red mud. CLEAN – Soil, Air, Water, 39, 972–979.
Parimaladevi, P., & Venkateswaran, V. (2001). Kinetics, thermodynamics and isotherm modeling of adsorption of triphenylmethane dyes (methyl violet, malachite green and magenta II) on to fruit waste. Journal of Applied Technology in Environmental Sanitation, 3, 273–283.
Pratt, K. C., & Christoverson, V. (1982). Hydrogenation of a model hydrogen-donor system using activated red mud catalyst. Fuel, 61, 460–462.
Robinson, R., Chandran, B., & Nigam, P. (2001). Removal of dyes from a synthetic textile dye effluent by biosorption on apple pomace and wheat straw. Water Research, 36, 2824–2830.
Robinson, T., Chandra, P., & Nigam, P. (2002). Removal of dyes from a synthetic textile dye effluent by biosorption on apple pomace and wheat straw. Water Research, 36, 2824–2830.
Rozada, F., Calvo, L. F., Garcia, A. I., Martin-Villarcota, J., & Otero, M. (2002). Dye adsorption by sewage sludge based activated carbons in batch and fixed bed systems. Bioresource Technology, 87, 221–230.
Senthilkumar, P., & Kirthika, K. (2009). Equilibrium and kinetic study of adsorption of nickel from aqueous solution onto bael tree leaf powder. Journal of Engineering Science and Technology, 4, 351–363.
Shing, J. S. (1997). Method of activation of Red Mud, US Patent 4017425.
Snigdha, S., Vidya, S., & Batra. (2012). Modification of red mud by acid treatment and its application for CO removal. Journal of Hazardous Materials, 203–204, 264–273.
Soner, H., & Altundogan, S. (2000). Arsenic removal from aqueous solutions by adsorption on red mud. Waste Management, 20, 761–767.
Sujana, M. G., Thakur, R. S., & Rao, S. B. (1998). Removal of fluoride from aqueous solution by using alum sludge. Journal of Colloid and Interface Science, 206, 94–101.
Tor, A., & Cengeloglu, Y. (2006). Removal of congo red from aqueous solution by adsorption onto acid activated red mud. Journal of Hazardous Materials, 138, 409–415.
Walker, G. M., & Weatherley, L. R. (1999). Kinetics of acid dye adsorption on GAC. Water Research, 33, 1895–1899.
Wang, S., Boyjoo, Y., Choueib, A., & Zhu, Z. H. (2005). Removal of dyes from aqueous solution using fly ash and red mud. Water Research, 39, 129–138.
Weber, J. R. (1973). The prediction of the performance of activated carbon for water treatment. In: proceeding of the conference activated carbon in water treatment. Water research association. University of Reading, UK, 53–71.
Weber, W. J., & Morris, J. C. (1963). Preliminary appraisal of advanced waste treatment process. Proceedings of International Conference Advances in Water Polution Research, 2, 231–241.
Wong, Y. C., Szeto, Y. S., Cheung, W. H., & McKay, G. (2004). Adsorption of acid dyes on chitosan–equilibrium isotherm analyses. Process Biochemistry, 39, 695–704.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ratnamala, G.M., Shetty, K.V. & Srinikethan, G. Removal of Remazol Brilliant Blue Dye from Dye-Contaminated Water by Adsorption Using Red Mud: Equilibrium, Kinetic, and Thermodynamic Studies. Water Air Soil Pollut 223, 6187–6199 (2012). https://doi.org/10.1007/s11270-012-1349-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11270-012-1349-4