Abstract
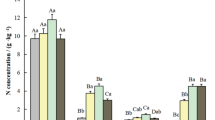
Attempts were made to quantify the carbon and nitrogen pools in a monospecific and pioneer mangrove stand of Kandelia obovata Sheue, Liu & Yong, Okinawa Island, Japan. The leaf C and N concentrations on a leaf area basis decreased with increasing PPFD (Photosysthetic Photon Flux Density). The total C and N stocks in foliage were estimated as 3.55 Mg ha–1 and 0.105 Mg ha–1, respectively. The bark (45.6–48.6% for C and 0.564–0.842% for N) contained significantly higher amount of C (P < 0.05) and N (P < 0.01) than wood (46.2–47.8% for C and 0.347–0.914% N). The total C stock of stem was 23.2 Mg ha–1 in wood and 8.33 Mg ha–1 in bark, and the total N stock was 0.222 Mg ha–1 in wood and 0.116 Mg ha–1 in bark. The root wood (37.1–45.0%) contained significantly higher amount of C than root bark (35.4–40.7%) (P < 0.01). The total C stock of root was 14.2 Mg ha–1 in wood and 12.6 Mg ha–1 in bark, and the total N stock of root was 0.157 Mg ha–1 in wood and 0.155 Mg ha–1 in bark. The soil organic C and total N stocks within 1 m soil depth were estimated as 57.3 Mg ha–1 and 2.73 Mg ha–1, respectively. The C pool in aboveground biomass (35.1 Mg ha–1) was 1.3 times as large as that in belowground biomass (26.9 Mg ha–1). However, the soil organic C pool (57.3 Mg ha–1) was similar to the total C pool (62.0 Mg ha–1) of vegetation, indicating that the mangrove stored a large part of production in the soil. About 50% of the C was in the soil. The N pool in aboveground biomass (0.442 Mg ha–1) was 1.4 times as large as that in belowground biomass (0.312 Mg ha–1). The soil N stock was 3.3 times as large as the biomass N stock (0.754 Mg ha–1).







Similar content being viewed by others
References
Alongi DM (1996) The dynamics of benthic nutrient pools and fluxes in tropical mangrove forests. J Mar Res 54:123–148
Alongi DM, Boto KG, Robertson AI (1992) Nitrogen and phosphorus cycles. In: Robertson AI, Alongi DM (eds) Tropical mangrove ecosystems. American Geophysical Union, Washington, pp 251–292
Alongi DM, Clough BF, Dixon P, Tirendi F (2003) Nutrient partitioning and storage in arid-zone forests of the mangroves Rhizophora stylosa and Avicennia marina. Trees 17:51–60
Alongi DM, Sasekumar A, Chong VC, Pfitzner J, Trott LA, Tirendi F, Dixon P, Brunskill GJ (2004a) Sediment accumulation and organic material flux in a managed mangrove ecosystem: estimates of land–ocean–atmosphere exchange in peninsular Malaysia. Mar Geol 208:383–402
Alongi DM, Tirendi F, Clough BF (2000) Below-ground decomposition of organic matter in forests of the mangroves Rhizophora stylosa and Avicennia marina along the arid coast of Western Australia. Aquat Bot 68:97–122
Alongi DM, Trott LA, Wattayakorn G, Clough BF (2002) Below-ground nitrogen cycling in relation to net canopy production in mangrove forests of southern Thailand. Mar Biol 140:855–864
Alongi DM, Wattayakorn G., Pfitzner J, Tirendi F, Zagorskis I, Brunskill GJ, Davidson A, Clough BF (2001) Organic carbon accumulation and metabolic pathways in sediments of mangrove forests in southern Thailand. Mar Geol 179:85–103
Alongi DM, Wattayakorn AG, Tirendi F, Dixon P (2004b) Nutrient capital in different aged forests of the mangrove Rhizophora apiculata. Botanica Marina 47:116–124
Ball MC, Critchley C (1982) Photosynthetic responses to irradiance by the grey mangrove, Avicennia marina, grown under different light regimes. Plant Physiol 70:1101–1106
Bouillon S, Dahdouh-Guebas F, Rao AVVS, Koedam N, Dehairs F (2003) Sources of organic carbon in mangrove sediments: variability and possible ecological implications. Hydrobiologia 495:33–39
Chen R, Twilley RR (1999) A simulation model of organic matter and nutrient accumulation in mangrove wetland soils. Biogeochemistry 44:93–118
Chhabra A, Palria S, Dadhwal VK (2003) Soil organic carbon pool in Indian forests. For Ecol Manage 173:187–199
Davidson EA, Trumbore SE, Amundson R (2000) Soil warming and organic carbon content. Nature 408:789–790
De Kovel CGF, Van Mierlo AJEM, Wilms YJO, Berendse F (2000) Carbon and nitrogen in soil and vegetation at sites differing in successional age. Plant Ecol 149:43–50
Dixon RK, Brown S, Houghton RA, Solomon AM, Trexler MC, Wisniewski J (1994) Carbon pools and flux of global forest ecosystems. Science 263:185–190
Duarte CM, Cebrián J (1996) The fate of marine autotrophic production. Limnol Oceanogr 41:1758–1766
Duarte CM, Geertz-Hansen O, Thampanya U, Terrados J, Fortes MD, Kamp-Nielsen NL, Borum J, Boromthanarath S (1998) Relationship between sediment conditions and mangrove Rhizophora apiculata seedling growth and nutrient status. Mar Ecol Prog Ser 175:277–283
Finér L, Mannerkoski H, Piirainen S, Starr M (2003) Carbon and nitrogen pools in an old-growth, Norway spruce mixed forest in eastern Finland and changes associated with clear-cutting. For Ecol Manage 174:51–63
Fujimoto K (2004) Below-ground carbon sequestration of mangrove forests in the Asia-Pacific region. In: Vannucci M (ed) Mangrove management and conservation. United Nations University Press, New York, pp 138–146
Gleason SM, Ewel KC (2002) Organic matter dynamics on the forest floor of a micronesian mangrove forest: an investigation of species composition shifts. Biotropica 34:190–198
Golley F, Odum HT, Wilson RF (1962) The structure and metabolism of Puerto Rican red mangrove forest in May. Ecology 43:9–19
Gong WK, Ong JE (1990) Plant biomass and nutrient flux in a managed mangrove forest in Malaysia. Estuar Coast Shelf Sci 31:519–530
Hagihara A, Ichikawa K, Hozumi K (1982) Vertical distributions of C and N concentrations and seasonal changes in foliage C and N in a Larix leptolepis stand (in Japanese). Tran 30th Mtg Chuba Br 37–40
Hart PBS, Clinton PW, Allen RB, Nordmeyer AH, Evans G (2003) Biomass and macro-nutrients (above- and below-ground) in a New Zealand beech forest ecosystem: implications for carbon storage and sustainable forest management. For Ecol Manage 174:281–294
Hogarth PJ (1999) The biology of mangroves. Oxford University Press, New York
Holguin G, Vazquez P, Bashan Y (2001) The role of sediment microorganisms in the productivity, conservation, and rehabilitation of the mangrove ecosystems: an overview. Biol Fertil Soils 33:265–278
Hosokawa T, Tagawa H, Chapman VJ (1977) Mangals of Micronesia, Taiwan, Japan, the Philippines and Oceania. In: Chapman VJ (ed) Wet coastal ecosystems. Elsevier, Amsterdam, pp 271–291
Hughes RF, Kauffman JB, Jaramillo VJ (1999) Biomass, carbon, and nutrient dynamics of secondary forests in a humid tropical region of México. Ecology 80:1892–1907
Islam MS, Shokita S, Mfilinge PL, Tsuchiya M (2004) Status of organic and inorganic nutrients in waters and sediments at the habitat of the mangrove sesarmid crabs from the Ryukyu Islands, Japan. Bull Fac Sci Univ Ryukyus 78:327–356
Jaramillo VJ, Kauffman JB, Rentería-Rodríguez L, Cummings DL, Ellingson LJ (2003) Biomass, carbon, and nitrogen pools in Mexican tropical dry forest landscapes. Ecosystems 6:609–629
Jennerjahn TC, Ittekkot V (2002) Relevance of mangroves for the production and deposition of organic matter along tropical continental margins. Naturwissenschaften 89:23–30
Jobbágy EG, Jackson RB (2000) The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol Appl 10:423–436
Khan MNI, Suwa R, Hagihara A, Ogawa K (2004) Interception of photosynthetic photon flux density in a mangrove stand of Kandelia candel (L.) Druce. J For Res 9:205–210
Kira T (1991) Forest ecosystems of east and southeast Asia in a global perspective. Ecol Res 6:185–200
Komiyama A, Havanond S, Srisawatt W, Mochida Y, Fujimoto K, Ohnishi T, Ishihara S, Miyagi T (2000) Top/root biomass ratio of a secondary mangrove (Ceriops tagal (Perr.) C.B. Rob.) forest. For Ecol Manage 139:127–134
Komiyama A, Ogino K, Aksornkoae S, Sabhasri S (1987) Root biomass of a mangrove forest in southern Thailand. 1. Estimation by the trench method and the zonal structure of root biomass. J Trop Ecol 3:97–108
Kristensen E, Andersen F, Kofoed LH (1988) Preliminary assesment of benthic community metabolism in a south-east Asian mangrove swamp. Mar Ecol Prog Ser 48:137–145
Kurachi N, Hagihara A, Hozumi K (1986) Evaluation of the light interception by non-photosynthetic organs in a Larix leptolepis plantation. Ecol Res 1:173–183
Lacerda LD, Ittekkot V, Patchineelam SR (1995) Biogeochemistry of mangrove soil organic matter: a comparison between Rhizophora and Avicennia soils in south-eastern Brazil. Estuar Coast Shelf Sci 40:713–720
Machiwa JF, Hallberg RO (2002) An empirical model of the fate of organic carbon in a mangrove forest partly affected by anthropogenic activity. Ecol Model 147:69–83
Marchand C, Baltzer F, Lallier-Vergès E, Albéric P (2004) Pore-water chemistry in mangrove sediments: relationship with species composition and developmental stages (French Guiana). Mar Geol 208:361–381
Marchand C, Lallier-Vergès E, Baltzer F (2003) The composition of sedimentary organic matter in relation to the dynamic features of a mangrove-fringed coast in French Guiana. Estuar Coast Shelf Sci 56:119–130
Matsui N (1998) Estimated stocks of organic carbon in mangrove roots and sediments in Hinchinbrook Channel, Australia. Mangr Salt Mars 2:199–204
Mfilinge PL, Atta N, Tsuchiya M (2002) Nutrient dynamics and leaf litter decomposition in a subtropical mangrove forest at Oura Bay, Okinawa, Japan. Trees 16:172–180
Middelburg JJ, Nieuwenhuize J, Slim FJ, Ohowa B (1996) Sediment biogeochemistry in an East African mangrove forest (Gazi Bay, Kenya). Biogeochemistry 34:133–155
Monsi M, Saeki T (1953) Über den Lichtfaktor in den pflanzengesellschaften und seine Bedeutung für die Stoffproduktion. Jpn J Bot 14:22–52
Mumby PJ, Edwards AJ, Arias-González JE, Lindeman KC, Blackwell PG, Gall A, Gorczynska MI, Harborne AR, Pescod CL, Renken H, Wabnitz CCC, Llewellyn G (2004) Mangroves enhance the biomass of coral reef fish communities in the Caribbean. Nature 427:533–536
Nakasuga T, Ôyama H, Haruki M (1974) Studies on the mangrove community. I. The distribution of the mangrove community in Japan (in Japanese with English summary). Jpn J Ecol 24:237–246
Parkinson RW, DeLaune RD, White JR (1994) Holocene sea-level rise and the fate of mangrove forests within the wider Caribbean region. J Coastal Res 10:1077–1086
Patenaude GL, Briggs BDJ, Milne R, Rowland CS, Dawson TP, Pryor SN (2003) The carbon pool in a British semi-natural woodland. Forestry 76:109–119
Pelegrí SP, Rivera-Monroy VH, Twilley RR (1997) A comparison of nitrogen fixation (acetylene reduction) among three species of mangrove litter, sediments, and pneumatophores in south Florida, USA. Hydrobiologia 356:73–79
Ramanathan AL, Subramanian V, Ramesh R, Chidambaram S, James A (1999) Environmental geochemistry of the Pichavaram mangrove ecosystem (tropical), southeast coast of India. Environ Geol 37:223–233
Rapp M, Regina IS, Rico M, Gallego HA (1999) Biomass, nutrient content, litterfall and nutrient return to the soil in Mediterranean oak forests. For Ecol Manage 119:39–49
Robertson AI, Alongi DM, Boto KG (1992) Food chains and carbon fluxes. In: Robertson AI, Alongi DM (eds) Tropical mangrove ecosystems. American Geophysical Union, Washington, pp 293–326
Saintilan N (1997a) Above- and below-ground biomass of mangroves in a sub-tropical estuary. Mar Freshwater Res 48:601–604
Saintilan N (1997b) Above- and below-ground biomass of two species of mangrove on the Hawkesbury River estuary, New South Wales. Mar Freshwater Res 48:147–152
Sherman RE, Fahey TJ, Howarth RW (1998) Soil-plant interactions in a neotropical mangrove forest: iron, phosphorus and sulfur dynamics. Oecologia 115:553–563
Sheue C, Liu H, Yong WH. 2003. Kandelia obovata (Rhizophoraceae), a new mangrove species from Eastern Asia. Taxon 52:287–294
Stinson G, Freedman B (2001) Potential for carbon sequestration in Canadian forests and agroecosystems. Mitigation Adapt Strat Global Change 6:1–23
Suzuki E, Tagawa H (1983) Biomass of a mangrove forest and a sedge marsh on Ishigaki Island, south Japan. Jpn J Ecol 33:231–234
Takeuchi T, Sugaya T, Kanazashi A, Yoshimaru H, Katsuta M (2001) Genetic diversity of Kandelia candel and Bruguiera gymnorrhiza in the southwest Islands, Japan. J For Res 6:157–162
Tabuchi R, Ogino K, Aksornkoae S, Sabhasri S (1983) Fine root amount of mangrove forest: a preliminary survey. Ind J Plant Sci 1:31–41
Tam NFY, Wong YS (1998) Variations of soil nutrient and organic matter content in a subtropical mangrove ecosystem. Water Air Soil Poll 103:245–261
Tamai S, Nakasuga T, Tabuchi R, Ogino K (1986) Standing biomass of mangrove forests in southern Thailand. J Jpn For Soc 68:384–388
Toma T, Ogino K (1995) Soil water movement of a mangrove forest in Halmahera Island, east Indonesia. Tropics 4:187–200
Woitchik AF, Ohowa B, Kazungu JM, Rao RG, Goeyens L, Dehairs F (1997) Nitrogen enrichment during decomposition of mangrove leaf litter in an east African coastal lagoon (Kenya): relative importance of biological nitrogen fixation. Biogeochemistry 39:15–35
Yamamuro M, Kayanne H (1995) Rapid direct determination of organic carbon and nitrogen in carbonate-bearing sediments using a Yanaco MT-5 CHN analyzer. Limnol Oceanogr 40:1001–1005
Yim YJ, Ogawa H, Kira T (1969) Light interception by stems in plant communities. Jpn J Ecol 19:233–238
Yoda K, Kira T (1969) Comparative ecological studies on three main types of forest vegetation in Thailand. V. Accumulation and turnover of soil organic matter, with notes on altitudinal soil sequence on Khao (Mt.) Luang, Peninsular Thailand. Nat Life SE Asia 6:83–112
Acknowledgements
We are grateful to Dr. L. Alhamd and Mr. S.M. Feroz who provided invaluable assistance for the collection of data. We also thank the Ministry of Environment, Japan, for access to the wildlife sanctuary, and Tomigusuku Community for permitting us the use of their land. This study was partially supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (nos. 16201009 and 16651009), and by the 21st Century COE program of University of the Ryukyus.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khan, M.N.I., Suwa, R. & Hagihara, A. Carbon and nitrogen pools in a mangrove stand of Kandelia obovata (S., L.) Yong: vertical distribution in the soil–vegetation system. Wetlands Ecol Manage 15, 141–153 (2007). https://doi.org/10.1007/s11273-006-9020-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11273-006-9020-8