Abstract
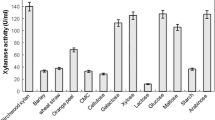
A halophilic and alkali-tolerant Chromohalobacter sp. TPSV 101 with an ability to produce extracellular halophilic, alkali-tolerant and moderately thermostable xylanase was isolated from solar salterns. Identification of the bacterium was done based upon biochemical tests and 16S rRNA sequence. The culture conditions for higher xylanase production were optimized with respect to NaCl, pH, temperature, substrates and metal ions and additives. Maximum xylanase production was achieved in the medium with 20% NaCl, pH-9.0 at 40°C supplemented with 1% (w/v) sugarcane bagasse and 0.5% feather hydrolysate as carbon and nitrogen sources. Sugarcane bagasse (250 U/ml) and wheat bran (190 U/ml) were the best inducer of xylanase when used as carbon source as compared to xylan (61 U/ml). The xylanase that was partially purified by protein concentrator had a molecular mass of 15 kDa approximately. The xylanase from Chromohalobacter sp. TPSV 101 was active at pH 9.0 and required 20% NaCl for optimal xylanolytic activity and was active over a broad range of temperature 40–80°C with 65°C as optimum. The early stage hydrolysis products of sugarcane bagasse were xylose and xylobiose, after longer periods of incubation only xylose was detected.






Similar content being viewed by others
References
Adams MWW, Perler FB, Kelly RM (1995) Extremozymes-expanding the limits of biocatalysis. Biotechnol 13:662–668. doi:10.1038/nbt0795-662
Amaya-Delgado L, Vega-Estrada J, Flores-Cotera LB, Dendooven L, Hidalgo-Lara ME, Montes-Horcasitas MC (2006) Induction of xylanases by sugar cane bagasse at different cell densities of Cellulomonas flavigena. Appl Microbiol Biotechnol 70:477–481. doi:10.1007/s00253-005-0096-5
Bataillon M, Nunes Cardiali AP, Castillon N, Duchiron F (2000) Purification and characterization of a moderately thermostable xylanase from Bacillus sp. strain SPS-0. Enzyme Microb Technol 26:187–192. doi:10.1016/S0141-0229(99)00143-X
Beg QK, Bhushan B, Kapoor M, Hondal GS (2000) Production and characterization of thermostable xylanase and pectinase from Streptomyces sp. QG-11-3. J Ind Microbiol Biotechnol 24:396–402. doi:10.1038/sj.jim.7000010
Beg QK, Kapoor M, Mahajan L, Hondal GS (2001) Microbial xylanases and their industrial applications: a review. Appl Microbiol Biotechnol 56:326–338. doi:10.1007/s002530100704
Biely P (1985) Microbial xylanolytic systems. Trends Biotechnol 3:286–290. doi:10.1016/0167-7799(85)90004-6
Cadenas Q, Engel PC (1994) Activity staining of halophilic enzymes: substitution of salt with zwitterions in non-denaturing electrophoresis. Biochem Mol Biol Int 33:785–792
Damaso MCT, De Castro AM, Castro RM, Andrade CMMC, Pereira N Jr (2004) Application of Xylanase from Thermomyces lanuginosus IOC-4145for Enzymatic Hydrolysis of Corncob and Sugarcane Bagasse. Appl Biochem Biotechnol 113:1003–1012
Dekker RFH, Richards GN (1975) Purification, properties, and mode of action of hemicellulose 1 by Ceratocystis paradoxa. Carbohydr Res 39:97–114. doi:10.1016/S0008-6215(00)82642-7
Ding CH, Jiang XT, Li LT, Kusakabe I (2004) High activity xylanase production by Streptomyces olivaceoviridis E-86. World J Microbiol Biotechnol 20:7–10. doi:10.1023/B:WIBI.0000013278.24679.ed
Flam F (1994) The chemistry of life at margins. Science 265:471–472. doi:10.1126/science.8036489
Fukushima T, Mizuki T, Akira Inoue AE, Usami R (2005) Organic solvent tolerance of halophilic α-amylase from a Haloarchaeon, Haloarcula sp strain S-1. Extremophiles 9:85–89. doi:10.1007/s00792-004-0423-2
Gokhale DV, Patil SG, Bastawde KB (1998) Potential application of yeast cellulase-free xylanase in agro waste material treatment to remove hemicellulose fractions. Bioresour Technol 63:187–191. doi:10.1016/S0960-8524(97)00062-X
Gupta S, Bhushan B, Hoondal GS, Kuhad RC (2001) Improved xylanase production from a haloalkaliphilic Staphylococcus sp. SG-13 using inexpensive agricultural residues. World J Microbiol Biotechnol 17:5–8. doi:10.1023/A:1016691205518
Huang L, Hseu TH, Wey TT (1991) Purification and characterization of an endoxylanase from Trichoderma koningii G-39. Biochem J 278:329–333
Hutcheon GW, Nishi V, Albert B (2005) Characterization of a highly stable α-amylase from the halophilic archaeon Haloarcula hispanica. Extremophiles 9:487–492. doi:10.1007/s00792-005-0471-2
Jatinder K, Chadha BS, Saini HS (2006) Optimization of culture conditions for production of cellulases and xylanases by Scytalidium thermophilum using Response Surface Methodology. World J Microbiol Biotechnol 22:169–176. doi:10.1007/s11274-005-9015-2
Karbalaei-Heidari HR, Ziaee AA, Amoozegar MA (2007) Purification and biochemical characterization of a protease secreted by the Salinivibrio sp. Strain AF-2004 and its behavior in organic solvents. Extremophiles 11:237–243. doi:10.1007/s00792-006-0031-4
Margesin R, Schinner F (2001) Potential of halotolerant and halophilic microorganisms for biotechnology. Extremophiles 5:73–83. doi:10.1007/s007920100184
Miller G (1959) Use of dinitrosalicylic acid reagent for determination of reducing sugars. Anal Chem 31:426–428. doi:10.1021/ac60147a030
Puls J, Poutanen K (1989) Mechanism of enzymic hydrolysis of hemicelluloses (xylans) and producers for determination of the activities involved. In: Coughlan MP (ed) Enzyme systems for lignocellulosic degradation. Elsevier, London, pp 151–165
Reilly PJ (1981) Xylanases: structure and function. Basic Life Sci 18:111–129
Silveira FQP, Melo IS, Filho EXF (1997) Carbohydrate-hydrolysing enzyme activity production by solid-state cultures of Trichoderma harzianum strains. Rev Microbiol 28:152–156
Sterner R, Liebl W (2001) Thermophilic adaptation of proteins. Crit Rev Biochem Mol Biol 36:39–106. doi:10.1080/20014091074174
Ventosa A, Nieto JJ (1995) Biotechnological applications and potentialities of halophilic microorganisms. World J Microbiol Biotechnol 11:85–94. doi:10.1007/BF00339138
Ventosa A, Nieto JJ, Oren A (1998) Biology of moderately halophilic aerobic bacteria. Microbiol Mol Biol Rev 62:504–544
Vidyasagar M, Prakash BS, Sreeramulu K (2006) Optimization of cultural conditions for the production of haloalkaliphilic thermostable protease from an extremely halophilic archaeon Halogeometricum sp. TSS101. Lett Appl Microbiol 43:385–391. doi:10.1111/j.1472-765X.2006.01980.x
Vidyasagar M, Prakash S, Jayalakshmi SK, Sreeramulu K (2007) Optimization of culture conditions for the production of halothermophilic protease from halophilic bacterium Chromohalobacter sp. TVSP101. World J Microbiol Biotechnol 23:655–662. doi:10.1007/s11274-006-9279-1
Vieille C, Liebl W (2001) Hyperthermophilic enzymes; sources, uses, and molecular mechanisms for thermostability. Microbiol Mol Biol Rev 65:1–42. doi:10.1128/MMBR.65.1.1-43.2001
Wainø M, Ingvorsen K (2003) Production of β-xylanase and β-xylosidase by the extremely halophilic archaeon Halorhabdus utahensis. Extremophiles 7:87–93
Wejse PL, Ingvorsen K, Mortensen KK (2003a) Purification and characterization of two extremely halotolerant xylanases from a novel halophilic bacterium. Extremophiles 7:423–431. doi:10.1007/s00792-003-0342-7
Wejse PL, Ingvorsen K, Mortensen KK (2003b) Xylanase production by a novel halophilic bacterium increased 20-fold by response surface methodology. Enzyme Microb Technol 32:721–727. doi:10.1016/S0141-0229(03)00033-4
Acknowledgment
One of the authors (Prakash B.) thanks the Council of Scientific and Industrial Research (CSIR) New Delhi, India for providing financial assistance in the form of Senior Research Fellowship (SRF) during this work. File No. 09/450 (0026) 2k8 EMR-I.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Prakash, S., Veeranagouda, Y., Kyoung, L. et al. Xylanase production using inexpensive agricultural wastes and its partial characterization from a halophilic Chromohalobacter sp. TPSV 101. World J Microbiol Biotechnol 25, 197–204 (2009). https://doi.org/10.1007/s11274-008-9880-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-008-9880-6