Abstract
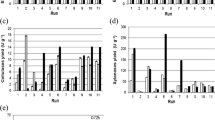
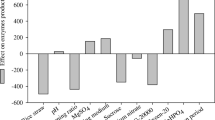
Two fungal strains were evaluated for β-N-acetylhexosaminidase production by solid state fermentation using different agro-industrial residues such as commercial wheat bran (CWB) and shrimp shell chitin waste (SSCW), of which Penicillium monoverticillium CFR 2 a local soil isolate showed significantly (P ≤ 0.001) higher β-N-acetylhexosaminidase activity on CWB medium as compared with the activity of Fusarium oxysporum CFR 8. Fermentation parameters such as incubation temperature, incubation time, initial moisture content and inoculum concentration were optimized by statistically designed experiments, using 3**(4–1) fractional factorial design of Response Surface Methodology. The high R2 (0.9512) observed during validation experiment showed the usefulness of the model. Highest level of enzyme activity (311.84 U/g IDS) was predicted at 75% (w/w) initial moisture content, 26 °C incubation temperature, 168 h incubation time and initial inoculum, at the highest concentration tested (2.95 ml spore suspension/5 g substrate). Statistical optimization yielded a 4.5 fold increase in β-N-acetylhexosaminidase activity. The crude β-N-acetylhexosaminidase showed optimum temperature of 57 ± 1 °C and pH of 3.6 and retained 50% activity after 1 h of incubation at 57 ± 1 °C. SDS–PAGE zymogram revealed crude enzyme was a monomer with an apparent molecular weight ~110 kDa. The crude enzyme formed 6.81 ± 0.03 mM/l of N-acetyl chitooligosaccharides from colloidal chitin in 24 h of incubation. HPLC analysis revealed hydrolysate contained 37.57% N-acetyl chitotriose and 62.43% N-acetyl chitohexose, indicating its potential for specific N-acetyl chitooligosaccharides production.






Similar content being viewed by others
References
Aiba SI (1994) Preparation of N-acetyl chitooligosaccarides by hydrolysis of chitosan with chitinase followed by N-acetylation. Carbohydr Res 265:323–328
Akira O (1988) Chitinase and β-N-acetyhesosaminidase from Pycnoporus cinnabarinus. In: Willis AW, Scott TK (eds) Methods in enzymology, vol 161. Academic Press Inc, London, pp 462–470
Akira O, Miho Y, Motoharu M, Takako I (1981) Purification and characterization of β-N-acetylhexosaminidase from Pycnoporus cinnabarinus. Agric Biol Chem 45(1):239–247
Aloise PA, Llumme M, Haynes CA (1996) N-acetyl D-glucosamnie production from chitin waste using chitinase from Serratia marcescens. In: Muzzarelli RAA (ed) Chitin enzymology, vol 2. European Chitin Society, Grottammare, pp 581–594
AOAC (2000) Official methods of analysis of AOAC International, vols. 1 and 2, 17th edn. AOAC International, Gaithersburg, MD
Araki Y, Ito E (1975) A pathway of chitosan formation in Mucor rouxii. Eur J Biochem 55:71–78
Bhaskar N, Suresh PV, Sakhare PZ, Sachindra NM (2007) Shrimp bio-waste fermentation with Pediococcus acidolactici CFR2182: optimization of fermentation conditions by response surface methodology and effect of optimized conditions on deproteinization/demineralization and carotenoid recovery. Enzymes Microbial Technol 40:1427–1434
Bidochka MJ, Tong KI, Khachatourians GG (1993) Partial Purification and characterization of chitosanase and exo-β-D-glucosaminidase produced by entamopathogenic fungus Beauveria bassiana. Can J Microbiol 39:40–45
Binod P, Chandran S, Pradeep S, George S, Asok P (2007) Fungal biosynthesis of endo-chitinase and chitobiase in solid state fermentation and their application for the production of N-acetyl-D-glucosamine from colloidal chitin. Bioresouce Technol 98:2742–2748
Chen X, Yin L, Guocheng D, Jian C (2005) Application of response surface methodology in medium optimization for spore production of Coniothyrium minitans in solid state fermentation. World J Microbiol Biotech 21:593–599
David BH (1990) One dimential polyacrylamide gel electrophoresis. In: Hames BD, Rickwood D (eds) Gel electrophoresis of protein. A practical approach, 2nd edn. Oxford University Press, New York, pp 1–147
Deepti A, Pankaj P, Tushar B, Shridhar P (2005) Alkaline protease production by a soil isolate of Beuveria feline under SSF condition: parameter optimization and application to soy protein hydrolysis. Process Biochem 40:1131–1136
Ghildyal NP, Lonsane BK, Sreekantiah KA, Sreenivasa MV (1985) Economics of submerged and solid state fermentations for the production of amyloglucosidase. J Food Sci Technol 22:171–176
Haki GD, Rakshi SK (2003) Developments in industrially important thermostable enzyme: a review. Bioresour Technol 89:17–34
Hirano S (1996) Chitin biotechnology applications. Biotechnol Ann Rev 2:237–258
Ilankovan P, Hein S, Ng C-H, Trung TS, Stevens WF (2006) Production of N-acetyl chitobiose from various chitin substrates using commercial enzymes. Carbohydr Res 63:245–250
Jeon YJ, Shahidi F, Kim SK (2000) Preparation of chitin and chitosan oligomers and their applications in physiological functional foods. Food Rev Int 16(2):159–176
Jung WJ, Souleimanov A, Park RD, Smith DL (2007) Enzymatic production of N-acetyl chitooligosaccharides by crude enzyme derived from Paenibacillus illiosensis KJA-424. Carbohydr Poly 67:256–259
Konstantinos G, Diomi M, Georgi N, Envangelos T, Poul C, Dimitris K, Basil JM (2004) Studies on N-acetyl-β-D-glucosaminidase produced by Fusarium oxysporum F3 grown in solid state fermentation. Process Biochem 39:599–605
Kumar S, Satyanarayana T (2004) Statistical optimization of a thermostable and natural glucoamylase production by thermophilic mold Thermomucor indicae-seudaticae in solid state fermentation. World J Microbiol Biotech 20:895–902
Laura RC, Maria del Carmen MC, Keiko S, Sergio R, Sergio H (2006) Enzymatic hydrolysis of chitin in the production of oligosaccharides using Lecanicillium fungicola chitinases. Process Biochem 41:1106–1110
Maria del Carmen MC, Yoyi M, Laura RC, Zaizy RP, Gustavo V, Keiko S (2008) Effect of moisture content in polyurethane foams as support for solid substrate fermentation of Lecanicillium lecanii on the production profiles of chitinases. Process Biochem 43:24–32
Nampoothiri KM, Baiju TV, Sandhya C, Sabu A, Szakacs G, Pandey A (2004) Process optimization for antifungal chitinase production by Trichoderma harzianum. Process Biochem 39:1583–1590
Nawani NN, Kapadnis BP (2005) Optimization of chitinase production using statistical based experimental designs. Process Biochem 40:651–660
Neetu D, Rupinder T, Ram PT, Gurinder SH (2005) Chitinase production in solid state fermentation by Enterobacter sp. NRG4 using statistical experimental design. Cur Microbiol 51:222–228
Nogawa M, Takahashi H, Kashiwagi A, Oshsima K, Okada H, Morikawa Y (1998) Purification and characterization of exo-β-D-glucosaminidase from a cellulolytic fungus Trichoderma reesei PC-3-7. Appl Environ Microbiol 64:890–895
Nopakarn R, Plikmol A, Yano S, Wakayama M, Tachiki T (2002) Utilization of shrimp shellfish waste a substrate for solid state cultivation of Aspergillus sp.S1-13: evaluation of a culture based on chitinase formation which is necessary for chitin assimilation. J Biosc Bioeng 93:550–556
Pandey A, Selvakumar P, Soccol CR, Nigam P (1999) Solid state fermentation for the production of industrial enzymes. Curr Sci 77:49–162
Pankaj P, Deepti A, Tushar B, Shridhar P (2005) Chitinase production by Beuveria feline RD 101: optimization of parameters under solid substrate fermentation conditions. World J Microbiol Biotech 21:93–95
Pera LM, Manjolli MVI, Baigori MD (1997) Purification and characterization of a thermostable and highly specific β-N-acetyl-D-glucosaminidase from Aspergillus nigar 419. Biotechnol Appl Biochem 26:183–187
Reetarani SP, Vandana G, Deshpande MV (2000) Chitinolytic enzymes: an exploration. Enzyme Microbial Technol 26:473–483
Ressing JL, Strominger JL, Leloir LF (1955) A modified colorimetric methods for estimation of N-acetyl amino sugars. J Bio Chem 217:959–962
Rodriguesz J, Copa-Patino JL, Perez-Leblic MI (1995) Purification and properties of a chitinase from Penicillium oxalatum autolyzate. Lett Appl Microbiol 20:46–49
Sashiwa H, Fugishima S, Yamano N, Kawasaki N, Nakayama A, Murtaki E, Hiraga K, Oda K, Aiba S (2003) Enzymatic production of N-acetyl-D-glucosamine from chitin. Degradation study of N-acetylchitooligosacchiade and the effect of mixing of crude enzyme by crude enzyme. Carbohydr Poly 51:391–395
Spinelli J, Lehman L, Wieg D (1974) Composition, processing and utilization of red crab (Pleuroncodes planipes) as an aquaculture feed ingredient. J Fish Res Board Canada 31:1025–1030
STATSOFT (1999) Statistica for windows. Statsoft Inc, Tulsa, UK
Suraini AA, Teoh LS, Noorjahan A, Neelam S, Kamarulzaman K (2008) Microbial degradation of chitin materials by Trichoderma virens UKM1. J Biol Sci 8(1):52–59
Suresh PV, Chandrasekaran M (1998) Utilization of prawn waste for chitinase production by the marine fungus Beauveria bassiana by solid state fermentation. World J Microbiol Biotech 14:655–660
Suresh PV, Chandrasekaran M (1999) Impact of processes parameters on chitinase production by an alkalophilic marine Beauveria bassiana in solid state fermentation. Process Biochem 34:257–267
Synowiecki J, Al-Khateeb NA (2003) Production, properties and some new applications of chitin and its derivatives. Cri Rev Food Sci Nitrition 43:145–171
Tanaka T, Fujiwara S, Nishikori S, Fukui T, Takagi M, Imanka T (1999) A unique chitinase with dual active sites and triple substrate binding sites from the hyperthermophilic Pyrococcus kodakaraensis KOD1. Appl Environ Microbiol 65:5338–5344
Tronsmo TA, Harman GE (1993) Detection and quantification of N-acetyl-β-D-glucosaminidase, chitobiosidase and endo-chitinase in solution and on gel. Anal Biochem 208:74–79
Vaiday R, Pranav V, Chhatpar HS (2003) Statistical optimization of medium components for the production of chitinase by Alcaligenes xylosoxydans. Enzyme Microb Technol 33:92–96
Vipul G, Tejas C, Pranav V, Chhatpar HS (2006) Statistical screening of medium components for the production of chitinase by the marine isolate Pantoea dispersa. Biochem Eng J 28:50–56
Wang SL, Lin TY, Yen YH, Liao FH, Chen YJ (2006) Bioconversion of shellfish chitin wastes for the production of Bacillus subtilis W-118 chitinase. Carbohydr Res 341:2507–2515
Woo C-J, Park H-D (2003) An extracellular Bacillus sp. chitinase for the production of chitotriose as a major chitinolytic product. Biotechnol Lett 25:409–412
Yoyi M, Gerardo S-C, Sergio R, Keiko S (2004) Production of β-N-acetylhexosaminidase of Verticillium lecanii by solid state and submerged fermentation utilizing shrimp waste silage as substrate and inducer. Process Biochem 39:665–671
Acknowledgments
Authors thank Director, CFTRI for his encouragement and permission to publish this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Suresh, P.V., Anil Kumar, P.K. & Sachindra, N.M. Thermoactive β-N-acetylhexosaminidase production by a soil isolate of Penicillium monoverticillium CFR 2 under solid state fermentation: parameter optimization and application for N-acetyl chitooligosaccharides preparation from chitin. World J Microbiol Biotechnol 27, 1435–1447 (2011). https://doi.org/10.1007/s11274-010-0596-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-010-0596-z