Abstract
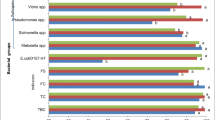
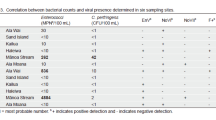
Pathogenic safety is drawing wide concern in water reclamation and reuse. In order to elucidate survive/fade of pathogens during the processes of wastewater treatment and reclamation, general indicators (fecal coliform and Escherichia coli), pathogenic bacteria (Salmonella and Shigella) and viruses (enterovirus, rotavirus and norovirus) were investigated in an A2O-MBR system. Attention was paid to their strengths from different sources, at various stages of the treatment, and in the product water. According to findings, black water was the main source for pathogens—at least 1–2-log higher in concentration than those from other sources. The preliminary treatment of wastewater by fine screens could bring about 0.2–0.4-log removal for almost all pathogens. The biological treatment units achieved almost identical removal (1.3–1.7-log) for bacteria and viruses. However, subsequent treatment in the membrane bioreactor showed varied removal for fecal coliform (4.7-log), E. coli (2.6-log) and the other pathogens (0.7–1.0-log), indicating that a high reduction of indicator bacteria may not imply equivalent removal of bacterial and viral pathogens. Chlorination was proved to be effective for eliminating all pathogens. In the artificial lake where the product water was stored, fecal coliform was not detected during the study period, but E. coli and pathogens were frequently detected, indicating that these bacterial and viral pathogens may be originating from non-fecal sources. On sunny summer days, the lake water could be bacteria-free due to sunlight radiation, but viruses were still detectable. Therefore, secondary disinfection may have to be adopted when the reclaimed water stored in such an open reservoir is supplied for strict reuse purposes.





Similar content being viewed by others
References
Ahmed W, Tucker J, Bettelheim KA, Neller R, Katoulia M (2007) Detection of virulence genes in Escherichia coli of an existing metabolic fingerprint database to predict the sources of pathogenic E. coli in surface waters. Water Res 41:3785–3791
Ana CL, Michel M, Andrey P, Javier M, Joan J, Rafael M, Lucena F (2008) Microbial indicators and pathogens: removal, relationships and predictive capabilities in water reclamation facilities. Water Res 42:4439–4448
Angelakis AN, Bontoux L (2001) Wastewater reclamation and reuse in European countries. Water Policy 3:47–59
Ayuso GN, Page D, Masciopinto C, Aharoni A, Salgot M, Wintgens T (2011) Quantifying the effect of Managed Aquifer Recharge on the microbiological human health risks of irrigating crops with recycled water. Agric Water Manag 99:93–102
Brookes JD, Antenucci J, Hipsey M, Burch MD, Ashbolt NJ, Ferguson C (2004) Fate and transport of pathogens in lakes and reservoirs. Environ Int 30:741–759
California Department of Health Services (1978) Wastewater reclamation criteria. California Admistrative code, title 22, division 4. California Department of Health Services, Berkeley
Chrysikopoulos CV, Aravantinou AF (2014) Virus attachment onto quartz sand: role of grain size and temperature. J Environ Chem Eng 2:796–801
Claudia SF, Wichern M, Horn H (2008) The impact of sunlight on inactivation of indicator microorganisms both in river water and benthic biofilms. Water Res 42:4771–4779
Dickenson JA, Sansalone JJ (2012) Distribution and disinfection of bacterial loadings associated with particulate matter fractions transported in urban wet weather flows. Water Res 46:6704–6714
Edberg S, Rice E, Karlin R, Allen M (2000) Escherichia coli: the best biological drinking water indicator for public health protection. J Appl Microbiol 88:106S–166S
Francy DS, Stelzer EA, Bushon RN, Brady AMG, Williston AG, Riddell KR, Borchardt MA, Spencer SK, Gellner TM (2012) Comparative effectiveness of membrane bioreactors, conventional secondary treatment, and chlorine and UV disinfection to remove microorganisms from municipal wastewaters. Water Res 46:4164–4178
George I, Crop P, Servais P (2002) Fecal coliform removal in wastewater treatment plants studied by plate counts and enzymatic methods. Water Res 36:2607–2617
Gilbride K (2014) Molecular Methods for the detection of waterborne pathogens. In: Bridle H (ed) Waterborne pathogens. Academic Press, Amsterdam, pp 231–290
Girones R, Ferrús MA, Alonso JL, Rodriguez MJ, Calgua B, de Corrêa AA, Hundesa A, Carratala A, Bofill-Mas S (2010) Molecular detection of pathogens in water—the pros and cons of molecular techniques. Water Res 44:4325–4339
Graczyk TK, Lucy FE (2007) Quality of reclaimed waters: a public health need for source tracking of wastewater-derived protozoan enteropathogens in engineered wetlands. T R Soc Trop Med Hyg 101:532–533
Guérineau H, Dorner S, Carrière A, McQuaid N, Sauvé S, Aboulfadl K, Hajj-Mohamad M, Prévost M (2014) Source tracking of leaky sewers: a novel approach combining fecal indicators in water and sediments. Water Res 58:50–61
Haaken D, Dittmar T, Schmalz V, Worch E (2014) Disinfection of biologically treated wastewater and prevention of biofouling by UV/electrolysis hybrid technology: influence factors and limits for domestic wastewater reuse. Water Res 52:20–28
Haramoto E, Yamada K, Nishida K (2011) Prevalence of protozoa, viruses, coliphages and indicator bacteria in groundwater and river water in the Kathmandu Valley, Nepa. T R Soc Trop Med Hyg 105:711–716
Harwood VJ, Levine AD, Scott TM, Chivukula V, Lukasik J, Farrah SR, Rose JB (2005) Validity of the indicator organism paradigm for pathogen reduction in reclaimed water and public health protection. Appl Environ Microbiol 71:3163–3170
Huang H, Young TA, Schwab KJ, Jacangelo JG (2012) Mechanisms of virus removal from secondary wastewater effluent by low pressure membrane filtration. J Membr Sci 409:1–8
Katayama H, Shimasaki A, Ohgaki S (2002) Development of a virus concentration method and its application to detection of enterovirus and norwalk Virus from coastal seawater. Appl Environ Microbiol 68:1033–1039
Kim T, Unno H (1996) The roles of microbes in the removal and inactivation of viruses in a biological wastewater treatment system. Water Sci Technol 33:243–250
Lewis GD, Metcaif TG (1988) Polyethylene glycol precipitation for recovery of pathogenic viruses, including hepatitis A virus and human rotavirus, from oyster, water, and sediment samples. Appl Environ Microbiol 54:1983–1988
Li D, Zeng S, Gu AZ, He M, Shi H (2013) Inactivation, reactivation and regrowth of indigenous bacteria in reclaimed water after chlorine disinfection of a municipal wastewater treatment plant. J Environ Sci-china 25:1319–1325
Manville D, Kleintop E, Miller B, Davis E, Mathewson J, Downs T (2001) Significance of indicator bacteria in a regionalized wastewater treatment plant and receiving waters. Int J Environ Pollut 15:461–466
Marti E, Monclús H, Jofre J, Rodriguez-Roda I, Comas J, Balcázar JL (2011) Removal of microbial indicators from municipal wastewater by a membrane bioreactor (MBR). Bioresour Technol 10:5004–5009
Nordgren J, Matussek A, Mattsson A, Svensson L, Lindgren PE (2009) Prevalence of norovirus and factors influencing virus concentrations during one year in a full-scale wastewater treatment plant. Water Res 43:1117–1125
Pang XL, Lee B, Boroumand N, Leblanc B, Preiksaitis JK, Ip CCY (2004) Increased detection of rotavirus using a real time reverse transcription-polymerase chain reaction -RT-PCR assay in stool specimens from children with diarrhea. J Med Virol 72:496–501
Percival S (2004) The survival and persistence of viruses in water. Microbiol Waterborne Dis 25:345–348
Percival SL, Williams DW (2014a) Salmonella. In: Percival SL, Yates MV, Williams DW, Chalmers RM, Gray NF (eds) Microbiology of waterborne diseases, 2nd edn. Academic Press, London, pp 209–222
Percival SL, Williams DW (2014b) Shigella. In: Percival SL, Yates MV, Williams DW, Chalmers RM, Gray NF (eds) Microbiology of waterborne diseases, 2nd edn. Academic Press, London, pp 223–236
Rabenau HF, Clarici AMK, Mu¨hlbauer G, Berger A, Vince A, Muller S, Daghofer E, Santner BI, Marth E, Kessler HH (2002) Rapid detection of enterovirus infection by automated RNA extraction and real-time fluorescence PCR. J Clin Virol 25:155–164
Rahn K, Grandis SAD, Clarke RC, McEwen SA, Galin JE, Ginocchio C, Gyles CL (1992) Amplification of an invA gene sequence of Salmonella typhimurium by polymerase chain reaction as a specific method of detection of Salmonella. Mol Cell Probe 6:271–279
Savichtcheva O, Okabe S (2006) Alternative indicators of fecal pollution: relations with pathogens and conventional indicators, current methodologies for direct pathogen monitoring and future application perspectives. Water Res 40:2463–2476
Sidhu JGR, Ho G, Unkovich I (2001) Role of indigenous microorganisms in suppression of Salmonella regrowth in composted biosolids. Water Res 35:913–920
Simmons FJ, Xagoraraki I (2011) Release of infectious human enteric viruses by full-scale wastewater utilities. Water Res 45:3590–3598
Simmons FJ, Kuo DHW, Xagoraraki I (2011) Removal of human enteric viruses by a full-scale membrane bioreactor during municipal wastewater processing. Water Res 45:2750–2793
Soda S, Ike M, Fujita M (1999) Adsorption of bacterial cells onto activated sludge flocs. J Biosci Bioeng 87:513–518
Soller JA, Schoen ME, Bartrand T, Ravenscroft JE, Ashbolt NJ (2010) Estimated human health risks from exposure to recreational waters impacted by human and non-human sources of faecal contamination. Water Res 44:4674–4691
Soule H, Genoulazi O, Benedicte GC, Chevallier P, Liu JX, Seigneurin JM (2000) Ultrafiltration and reverse transcription-polymerase chain reaction an efficient process for poliovirus, rotavirus and hepatitis a virus detection in water. Water Res 34:1063–1067
Stals A, Baert L, Botteldoornc N, Werbrouck H, Herman L, Uyttendaele M (2009) Multiplex real-time RT-PCR for simultaneous detection of GI/GII noroviruses and murine norovirus 1. J Virol Methods 161:247–253
State Environmental Protection Agency (2002) Water and wastewater monitoring analysis method. China Environ Sci Press, Bejing, pp 704–706
The state administration of quality supervision, inspection and quarantine of China (2002) The reuse of recycling water for urban—water quality standard for urban miscellaneous water consumption. GB/T 18920-2002
Theron J, Morar D, Preez MD, Brozel VS, Venter SN (2001) A sensitive seminested PCR method for the detection of Shigella in spiked environmental water samples. Water Res 35(4):869–874
Toze S, Bekele E, Page D, Sidhu J, Shackleton M (2010) Use of static quantitative microbial risk assessment to determine pathogen risks in an unconfined carbonate aquifer used for managed aquifer recharge. Water Res 44(4):1038–1049
US Environmental Protection Agency (2004) Guidelines for water reuse. EPA/625/R-04/108
Wen Q, Tutuka C, Keegan A, Jin B (2009) Fate of pathogenic microorganisms and indicators in secondary activated sludge wastewater treatment plants. J Environ Manag 90:1442–1447
Yates MV (2014a) Enterovirus. In: Percival SL, Yates MV, Williams DW, Chalmers RM, Gray NF (eds) Microbiology of waterborne diseases, 2nd edn. Academic Press, London, pp 493–504
Yates MV (2014b) Rotavirus. In: Percival SL, Yates MV, Williams DW, Chalmers RM, Gray NF (eds) Microbiology of waterborne diseases, 2nd edn. Academic Press, London, pp 523–527
Yates MV (2014c) Norovirus. In: Percival SL, Yates MV, Williams DW, Chalmers RM, Gray NF (eds) Microbiology of waterborne diseases, 2nd edn. Academic Press, London, pp 515–522
Yi L, Jiao W, Chen X, Chen W (2011) An overview of reclaimed water reuse in China. J Environ Sci-china 23:1585–1593
Zhang K, Farahbakhsh K (2007) Removal of native coliphages and coliform bacteria from municipal wastewater by various wastewater treatment processes: implications to water reuse. Water Res 41:2816–2824
Acknowledgments
This study was supported by the strategic China-Japan Joint Research Program on “S&T for Environmental Conservation and Construction of a Society with Less environmental Burden” (NSFC Grant No. 51021140002) and Program for Innovative Research Team in Shaanxi (PIRT) (Grant No. 2013KCT-13). The authors also acknowledge the support of Projects for Science and Technology in Xi’an (CX12160).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, J., Wang, X.C., Ji, Z. et al. Source identification of bacterial and viral pathogens and their survival/fading in the process of wastewater treatment, reclamation, and environmental reuse. World J Microbiol Biotechnol 31, 109–120 (2015). https://doi.org/10.1007/s11274-014-1770-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-014-1770-5