Abstract
Background, aim, and scope
Sorption of hydrophobic organic compounds (HOCs) to natural organic matter (NOM) is an important process that affects the transport, transformation, bioavailability, and fate of HOCs in the environment. Manufactured nanoparticles (NPs) such as nano-oxides will inevitably enter the environment in the processes of their production, transfer, and use and could be coated by the ubiquitous NOM. Thus, sorption of HOCs to NOM in the environment could be affected by the NP interactions with NOM. Furthermore, the toxicity of nano-oxides could be increased due to the adsorbed HOCs. Therefore, sorption of phenanthrene by nano-Al2O3 coated with humic acid (HA) was examined in this study to explore the possible effect of nanoparticles (NPs) on the environmental behavior of HOCs and the potential environmental and health risks of NPs.
Materials and methods
Four HAs were sequentially extracted with 0.1 mol/L NaOH from a peat soil. HAs, nano-Al2O3, and HA-coated nano-Al2O3 were characterized by techniques such as elemental analysis, solid-state 13C NMR, N2 surface area analysis, and zeta potential measurement. Adsorption isotherms of HAs by nano-Al2O3 and phenanthrene by HAs and HA-coated nano-Al2O3 were obtained using a batch equilibration technique at 25 ± 1°C. HA concentrations were measured by total organic carbon analysis. Phenanthrene concentrations were measured by liquid scintillation counting.
Results
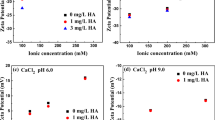
The adsorption maxima of HAs by nano-Al2O3 was one order of magnitude higher than that by soil inorganic minerals. Phenanthrene isotherms of HA-coated nano-Al2O3 were more nonlinear than that of their respective bulk HAs. Concentration-dependent organic carbon-normalized sorption coefficients (K′ oc) of phenanthrene by HA-coated nano-Al2O3 were lower than those for their respective bulk HAs, especially at relatively high concentrations.
Discussion
Isotherm nonlinearity of phenanthrene could be interpreted by a combination of partitioning accompanied by linear isotherm with adsorption accompanied by nonlinear isotherm. HA conformation changes during their adsorption on nano-Al2O3 could play an important role in phenanthrene sorption and were responsible for higher nonlinearity of phenanthrene isotherms and lower phenanthrene K′ oc on the adsorbed HAs than their respective bulk HAs. Adsorption of HA on nano-Al2O3 would form a more condensed HA state with higher π-polarity/polarizability and lower partitioning affinity than the respective bulk HA, leading to an increase of relative contribution of adsorption to the total sorption and more nonlinear phenanthrene isotherms in the adsorbed HA due to the increase in phenanthrene adsorption affinity and decrease in phenanthrene partitioning affinity.
Conclusions
Adsorption of HA on nano-Al2O3 was much higher than that on soil oxide minerals and could form a more condensed HA state with higher π-polarity/polarizability and lower partitioning affinity than the bulk HA, causing the significant difference in phenanthrene sorption between the adsorbed HA and the respective bulk HA. Therefore, once released in the environment, NPs such as nano-Al2O3 will strongly alter the environmental transport, fate, and bioavailability of HOCs and could be potentially more toxic due to the adsorbed toxic chemicals.
Recommendations and perspectives
Due to the high adsorption of HA on nano-Al2O3 and its significant effect on phenanthrene sorption, interactions of NOM with nano-oxides and their mechanistic relations with NOM conformation changes and HOC sorption merit further research. In addition, due to the higher sorption of phenanthrene on the HA-coated nano-Al2O3 than the pure counterpart, the effect of NOM and HOCs on the ecotoxicity of NPs should be addressed in the future.




Similar content being viewed by others
References
Aitken RJ, Chaudhry MQ, Boxall ABA, Hull M (2006) Manufacture and use of nanomaterials: current status in the UK and global trends. Occup Med 56:300–306
Chiou CT (2002) Partition and adsorption of organic contaminants in environmental systems. Wiley, New York
Feng XJ, Simpson AJ, Simpson MJ (2006) Investigating the role of mineral-bound humic acid in phenanthrene sorption. Environ Sci Technol 40:3260–3266
Giasuddin ABM, Kanel SR, Choi H (2007) Adsorption of humic acid onto nanoscale zerovalent iron and its effect on arsenic removal. Environ Sci Technol 41:2022–2027
Gu B, Schmitt J, Chen Z, Liang L, McCarthy JF (1994) Adsorption and desorption of natural organic matter on iron oxide: mechanisms and models. Environ Sci Technol 28:38–46
Gunasekara A, Xing B (2003) Sorption and desorption of naphthalene by soil organic matter: importance of aromatic and aliphatic components. J Environ Qual 32:240–246
Heinlaa M, Ivask A, Blinova I, Dubourguier H-C, Kahru A (2008) Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus. Chemosphere 71:1308–1316
Hickey JP, Passino-Reader DR (1991) Linear salvation energy relationships: ‘Rules of thumb’ for estimation of variable values. Environ Sci Technol 25:1753–1760
Huang WL, Schlautman MA, Weber WJ Jr (1996) A distributed reactivity model for sorption by soils and sediments. 5. The influence of near-surface characteristics in mineral domains. Environ Sci Technol 30:2993–3000
Huang W, Young T, Schlautman MA, Yu H, Weber WJ Jr (1997) A distributed reactivity model for sorption by soils and sediments. 9. General isotherm nonlinearity and applicability of the dual reactive domain model. Environ Sci Technol 31:1703–1710
Iorio M, Pan B, Capasso R, Xing B (2008) Sorption of phenanthrene by dissolved organic matter and its complex with aluminum oxide nanoparticles. Environ Pollut 156:1021–1029
Jones KD, Tiller CL (1999) Effect of solution chemistry on the extent of binding of phenanthrene by a soil humic acid: a comparison of dissolved and clay bound humic. Environ Sci Technol 33:580–587
Kang S, Xing B (2005) Phenanthrene sorption to sequentially extracted soil humic acids and humin. Environ Sci Technol 39:134–140
Kang S, Xing B (2008) Humic acid fractionation upon sequential adsorption onto goethite. Langmuir 24:2525–2531
Murphy EM, Zachara JM, Smith SC (1990) Influence of mineral-bound humic substances on the sorption of hydrophobic organic compounds. Environ Sci Technol 24:1507–1516
Murphy EM, Zachara JM, Smith SC, Phillips JL, Wietsma TW (1994) Interaction of hydrophobic organic compounds with mineral-bound humic substances. Environ Sci Technol 28:1291–1299
Nohynek GJ, Lademann J, Ribaud C, Roberts MS (2007) Grey goo on the skin? Nanotechnology, cosmetic and sunscreen safety. Toxicology 37:251–277
Nowack B, Bucheli TD (2007) Occurrence, behavior and effects of nanoparticles in the environment. Environ Pollut 150:5–22
Pang YX, Bao XJ (2002) Aluminum oxide nanoparticles prepared by water-in-oil microemulsions. J Mater Chem 12:3699–3704
Perminova IV, Grechishcheva NY, Petrosyan VS (1999) Relationships between structure and binding affinity of humic substances for polycyclic aromatic hydrocarbons: Relevance of molecular descriptors. Environ Sci Technol 33:3781–3787
Roco MC (2005) International perspective on government nanotechnology funding in 2005. J Nanopart Res 7:707–712
Salloum MJ, Chefetz B, Hatcher PG (2002) Phenanthrene sorption to aliphatic-rich natural organic matter. Environ Sci Technol 36:1953–1958
Wang KJ, Xing B (2005) Structural and sorption characteristics of adsorbed humic acid on clay minerals. J Environ Qual 34:342–349
Wang X, Lu J, Xu M, Xing B (2008) Sorption of pyrene by regular and nanoscaled metal oxide particles: influence of adsorbed organic matter. Environ Sci Technol 42:7267–7272
Xing B (2001) Sorption of anthropogenic organic compounds by soil organic matter: a mechanistic consideration. Can J Soil Sci 81:317–323
Xing B, Pignatello JJ (1997) Dual-mode sorption of low-polarity compounds in glassy poly(vinylchloride) and soil organic matter. Environ Sci Technol 31:792–799
Xing B, McGill WB, Dudas MJ (1994) Cross-correlation of polarity curves to predict partition coefficients of nonionic organic contaminants. Environ Sci Technol 28:1929–1933
Yang K, Zhu L, Lou B, Chen B (2005) Correlations of nonlinear sorption of organic solutes with soil/sediment physicochemical properties. Chemosphere 61:116–128
Yang K, Zhu L, Xing B (2006) Adsorption of polycyclic aromatic hydrocarbons by carbon nanomaterials. Environ Sci Technol 40:1855–1861
Acknowledgements
This work was supported by Program for New Century Excellent Talents in University of China (NCET–08–493), NSF of China (20777065 and 20737002), the Scientific Research Foundation for the Returned Overseas Chinese Scholars, Massachusetts Agricultural Experiment Station (MAS00973), the USDA (CSREES) National Research Initiative Competitive Grants Program (2005-35107-15278), and Massachusetts Water Resources Research Center (2007MA73B).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Christian Steinberg
Electronic supplementary material
This material is available via the Internet at www.springerlink.com.
ESM 1
(PDF 144 kb)
Rights and permissions
About this article
Cite this article
Yang, K., Zhu, L. & Xing, B. Sorption of phenanthrene by nanosized alumina coated with sequentially extracted humic acids. Environ Sci Pollut Res 17, 410–419 (2010). https://doi.org/10.1007/s11356-009-0163-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-009-0163-z