Abstract
Background, aim, and scope
The occurrence and fate of pharmaceuticals in the aquatic environment is recognized as one of the emerging issues in environmental chemistry and as a matter of public concern. Existing data tend to focus on the concentrations of pharmaceuticals in the aqueous phase, with limited studies on their concentrations in particulate phase such as sediments. Furthermore, current water quality monitoring does not differentiate between soluble and colloidal phases in water samples, hindering our understanding of the bioavailability and bioaccumulation of pharmaceuticals in aquatic organisms. In this study, an investigation was conducted into the concentrations and phase association (soluble, colloidal, suspended particulate matter or SPM) of selected pharmaceuticals (propranolol, sulfamethoxazole, meberverine, thioridazine, carbamazepine, tamoxifen, indomethacine, diclofenac, and meclofenamic acid) in river water, effluents from sewage treatment works (STW), and groundwater in the UK.
Materials and methods
The occurrence and phase association of selected pharmaceuticals propranolol, sulfamethoxazole, meberverine, thioridazine, carbamazepine, tamoxifen, indomethacine, diclofenac, and meclofenamic acid in contrasting aquatic environments (river, sewage effluent, and groundwater) were studied. Colloids were isolated by cross-flow ultrafiltration (CFUF). Water samples were extracted by solid-phase extraction (SPE), while SPM was extracted by microwave. All sample extracts were analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS) in the multiple reaction monitoring.
Results and discussion
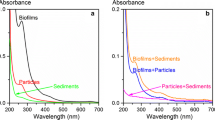
Five compounds propranolol, sulfamethoxazole, carbamazepine, indomethacine, and diclofenac were detected in all samples, with carbamazepine showing the highest concentrations in all phases. The highest concentrations of these compounds were detected in STW effluents, confirming STW as a key source of these compounds in the aquatic environments. The calculation of partition coefficients of pharmaceuticals between SPM and filtrate (observed partition coefficients, \( K_p^{\text{obs}} \), \( K_{\text{oc}}^{\text{obs}} \)), between SPM and soluble phase (intrinsic partition coefficients, \( K_p^{\text{int}} \), \( K_{\text{oc}}^{\text{int}} \)), and between colloids and soluble phase (K coc) showed that intrinsic partition coefficients \( \left( {K_p^{\text{int}},K_{\text{oc}}^{\text{int}}} \right) \) are between 25% and 96%, and between 18% and 82% higher than relevant observed partition coefficients values, and are much less variable. Secondly, K coc values are 3–4 orders of magnitude greater than \( K_{\text{oc}}^{\text{int}} \) values, indicating that aquatic colloids are substantially more powerful sorbents for accumulating pharmaceuticals than sediments. Furthermore, mass balance calculations of pharmaceutical concentrations demonstrate that between 23% and 70% of propranolol, 17–62% of sulfamethoxazole, 7–58% of carbamazepine, 19–84% of indomethacine, and 9–74% of diclofenac are present in the colloidal phase.
Conclusions
The results provide direct evidence that sorption to colloids provides an important sink for the pharmaceuticals in the aquatic environment. Such strong pharmaceutical/colloid interactions may provide a long-term storage of pharmaceuticals, hence, increasing their persistence while reducing their bioavailability in the environment.
Recommendations and perspectives
Pharmaceutical compounds have been detected not only in the aqueous phase but also in suspended particles; it is important, therefore, to have a holistic approach in future environmental fate investigation of pharmaceuticals. For example, more research is needed to assess the storage and long-term record of pharmaceutical residues in aquatic sediments by which benthic organisms will be most affected. Aquatic colloids have been shown to account for the accumulation of major fractions of total pharmaceutical concentrations in the aquatic environment, demonstrating unequivocally the importance of aquatic colloids as a sink for such residues in the aquatic systems. As aquatic colloids are abundant, ubiquitous, and highly powerful sorbents, they are expected to influence the bioavailability and bioaccumulation of such chemicals by aquatic organisms. It is therefore critical for colloids to be incorporated into water quality models for prediction and risk assessment purposes.




Similar content being viewed by others
References
Ashton D, Hilton M, Thomas KV (2004) Investigating the environmental transport of human pharmaceuticals to streams in the United Kingdom. Sci Total Environ 333:167–184
Badr EA, Tappin AD, Achterberg EP (2008) Distributions and seasonal variability of dissolved organic nitrogen in two estuaries in SW England. Mar Chem 110:153–164
Castiglioni S, Bagnati R, Fanelli R, Pomati F, Calamari D, Zuccato E (2006) Removal of pharmaceuticals in sewage treatment plants in Italy. Environ Sci Technol 40:357–36
Clara M, Strenn B, Kreuzinger N (2004) Comparison of the behaviour of selected micropollutants in a membrane bioreactor and a conventional wastewater treatment plant. Water Sci Technol 50:29–36
Drillia P, Stamatelatou K, Lyberatos G (2005) Fate and mobility of pharmaceuticals in solid matrices. Chemosphere 60:1034–1044
Fent K, Weston AA, Caminada D (2006) Review: ecotoxicology of human pharmaceuticals. Aquat Toxicol 76:122–159
Gentili A, Marchese PS (2005) Liquid chromatography-tandem mass spectrometry for performing confirmatory analysis of veterinary drugs in animal food products. Trends Anal Chem 24:704–733
Gooddy DC, Mathias SA, Harrison I, Lapworth DJ, Kim AW (2007) The significance of colloids in the transport of pesticides through Chalk. Sci Total Environ 385:262–271
Heberer T, Reddersen K, Mechlinski A (2002) From municipal sewage to drinking water: fate and removal of pharmaceutical residues in the aquatic environment in urban areas. Water Sci Technol 46:81–88
Hibberd A, Maskaoui K, Zhang Z, Zhou JL (2009) An improved method for the simultaneous analysis of phenolic and steroidal estrogens in water and sediment. Talanta 77:1315–1321
Holbrook RD, Love NG, Novak JT (2004) Sorption of 17β-estradiol, 17α-ethynylestradiol by colloid organic carbon derived from biological wastewater treatment systems. Environ Sci Technol 38:3322–3329
Jones N, Voulvoulis N, Lester JN (2007) The occurrence and removal of selected pharmaceutical compounds in a sewage treatment works utilising activated sludge treatment. Environ Pollut 145:738–744
Joss A, Keller E, Alder AC, Goebel A, McArdell CS, Ternes T, Siegrist H (2005) Removal of pharmaceuticals and fragrances in biological wastewater treatment. Water Res 39:3139–3152
Kasprzyk-Hordern B, Dinsdale RM, Guwy AJ (2008) Multiresidue methods for the analysis of pharmaceuticals, personal care products and illicit drugs in surface water and wastewater by solid-phase extraction and ultra performance liquid chromatography-electrospray tandem mass spectrometry. Anal Bioanal Chem 391:1293–1308
Kimura K, Hara H, Watanabe Y (2007) Elimination of selected acidic pharmaceuticals from municipal wastewater by an activated sludge system and membrane bioreactors. Environ Sci Technol 41:3708–3714
Liu R, Zhou JL, Wilding A (2004) Microwave-assisted extraction followed by gas chromatography-mass spectrometry for the determination of endocrine disrupting chemicals in river sediments. J Chromatogr A 1038:19–26
Liu R, Wilding A, Hibberd A, Zhou JL (2005) Partition of endocrine-disrupting chemicals between colloids and dissolved phase as determined by cross-flow ultrafiltration. Environ Sci Technol 39:2753–2761
Löffler D, Römbke R, Meller M, Ternes TA (2005) Environmental fate of pharmaceuticals in water/sediment systems. Environ Sci Technol 39:5209–5218
Maskaoui K, Hibberd A, Zhou JL (2007) Assessment of the interaction between aquatic colloids and pharmaceuticals facilitated by cross-flow ultrafiltration. Environ Sci Technol 41:8038–8043
Matamoros V, Bayona JM (2006) Elimination of pharmaceuticals and personal care products in subsurface flow constructed wetlands. Environ Sci Technol 40:5811–5816
Matamoros V, Arias C, Brix H, Bayona JM (2007) Removal of pharmaceuticals and personal care products (PPCPs) from urban wastewater in a pilot vertical flow constructed wetland and a sand filter. Environ Sci Technol 41:8171–8177
Miao XS, Bishay F, Chen M, Metcalfe CD (2004) Occurrence of antimicrobials in the final effluents of wastewater treatment plants in Canada. Environ Sci Technol 38:3533–3541
Miao XS, Yang JJ, Metcalfe CD (2005) Carbamazepine and its metabolites in wastewater and in biosolids in a municipal wastewater treatment plant. Environ Sci Technol 39:7469–7475
Nakada N, Tanishima T, Shinohara H, Kiri K, Takada H (2006) Pharmaceutical chemicals and endocrine disrupters in municipal wastewater in Tokyo and their removal during activated sludge treatment. Water Res 40:3297–3303
Nikolaou A, Meric S, Fatta D (2007) Occurrence patterns of pharmaceuticals in water and wastewater environments. Anal Bioanal Chem 387:1225–1234
Paxéus N (2004) Removal of selected non-steroidal anti-inflammatory drugs (NSAIDs), gemfibrozil, carbamazepine, beta-blockers, trimethoprim and triclosan in conventional wastewater treatment plants in five EU countries and their discharge to the aquatic environment. Water Sci Technol 50:253–260
Petrović M, Hernando MD, Diaz-Cruz MS, Barceló D (2005) Liquid chromatography-tandem mass spectrometry for the analysis of pharmaceutical residues in environmental samples: a review. J Chromatogr A 1067:1–14
Spongberg AL, Witter JD (2008) Pharmaceutical compounds in the wastewater process stream in Northwest Ohio. Sci Total Environ 397:148–157
Stein K, Ramil M, Fink G, Sander M, Ternes TA (2008) Analysis and sorption of psychoactive drugs onto sediments. Environ Sci Technol 42:6415–6423
Stumpf M, Ternes TA, Wilken R, Rodrigues SV, Baumann W (1999) Polar drug residues in sewage and natural waters in the State of Rio de Janeiro, Brazil. Sci Total Environ 225:135–141
Ternes TA (1998) Occurrence of drugs in German sewage treatment plants and rivers. Water Res 32:3245–3260
Tixier C, Singer HP, Oellers S, Muller SR (2003) Occurrence and fate of carbamazepine, clofibric acid, diclofenac, ibuprofen, ketoprofen, and naproxen in surface waters. Environ Sci Technol 37:1061–1068
Wiegel S, Aulinger A, Brockmeyer R, Harms H, Loffler J, Reincke H, Schmidt R, Stachel B, Tumpling WV, Wanke A (2004) Pharmaceuticals in the river Elbe and its tributaries. Chemosphere 57:107–126
Wilding A, Liu R, Zhou JL (2004) Validation of cross-flow ultrafiltration for sampling of colloidal particles from aquatic systems. J Colloid Interface Sci 280:102–112
Wilding A, Liu R, Zhou JL (2005) Dynamic behaviour of colloidal and dissolved organic matter through cross-flow ultrafiltration system. J Colloid Interface Sci 287:152–158
Zhang ZL, Zhou JL (2007) Simultaneous determination of various pharmaceutical compounds in water by solid-phase extraction-liquid chromatography-tandem mass spectrometry. J Chromatogr A 1154:205–213
Zhang Z, Hibberd A, Zhou JL (2008) Analysis of emerging contaminants in sewage effluent and river water: comparison between spot and passive sampling. Anal Chim Acta 607:37–44
Zhou JL, Liu R, Wilding A, Hibberd A (2007) Sorption of selected endocrine disrupting chemicals to different aquatic colloids. Environ Sci Technol 41:206–213
Zhou JL, Zhang ZL, Banks E, Grover D, Jiang JQ (2009) Pharmaceutical residues in wastewater treatment works effluents and their impact on receiving river water. J Hazard Mater 166:655–661
Acknowledgments
This work was funded by the European Union under FP6 (contract number MIF1-CT-2006-021556). We also thank Andrew Hibberd for help with sampling and colloidal isolation and Prof Eric Achterberg of University of Southampton for DOC analyses.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Thomas Braunbeck
Rights and permissions
About this article
Cite this article
Maskaoui, K., Zhou, J.L. Colloids as a sink for certain pharmaceuticals in the aquatic environment. Environ Sci Pollut Res 17, 898–907 (2010). https://doi.org/10.1007/s11356-009-0279-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-009-0279-1