Abstract
Purpose
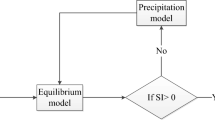
Both an optimization statistical model and a chemical thermodynamic equilibrium computer model were proposed to develop, improve, and optimize struvite precipitation process.
Methods and result
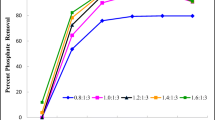
The NH4-N in synthetically prepared wastewater was removed using struvite precipitation technology. A quadratic statistical modeling, response surface methodology (RSM), was applied to investigate the improvement availability for high-level removal of ammonium-nitrogen by struvite precipitation. Then, a chemical equilibrium model, Visual MINTEQ, was used to calculate the equilibrium speciation and saturation index in aqueous solution and solid phases. In addition, the availability of Mg2+, NH +4 , and PO 3−4 ions as a function of pH was modeled. The predicted and experimental data indicated that the two models might describe the experiments well. The results showed that pH was an important parameter in ammonium-nitrogen removals at low initial NH4-N concentration. P/N molar ratio was a limiting factor on struvite precipitation at high initial NH4-N concentration.
Conclusion
Within the ranges of the investigated factors, Visual MINTEQ program can be proposed to predetermine the concentration of ammonium precipitated by struvite, and RSM can be used to predict total NH4-N removal by both struvite precipitation and ammonia volatilization from our investigated system operated at high pH and opened to the atmosphere.





Similar content being viewed by others
References
Ali MI, Schneider PA (2008) An approach of estimating struvite growth kinetic incorporating thermodynamic and solution chemistry, kinetic and process description. Chem Eng Sci 63:3514–3525
APHA (2005) Standard methods for the examination of water and wastewater. American Public Health Association, Washington, DC
Basakcilardan-Kabakci S, Ipekoglu AN, Talinli I (2007) Precipitation of urinary phosphate. Environ Eng Sci 24:1399–1408
Battistoni P, Pavan P, Prisciandaro M, Cecchi F (2000) Struvite crystallization: a feasible and reliable way to fix phosphorus in anaerobic supernatants. Water Res 34:3033–3041
Battistoni P, De Angelis A, Prisciandaro M, Boccadoro R, Bolzonella D (2002) P removal from anaerobic supernatants by struvite crystallization: long term validation and process modelling. Water Res 36:1927–1938
Çelen I, Buchanan JR, Burns RT, Bruce Robinson R, Raj Raman D (2007) Using a chemical equilibrium model to predict amendments required to precipitate phosphorus as struvite in liquid swine manure. Water Res 41:1689–1696
Doyle JD, Parsons SA (2002) Struvite formation, control and recovery. Water Res 36:3925–3940
Kim D, Kim J, Ryu HD, Lee SI (2009) Effect of mixing on spontaneous struvite precipitation from semiconductor wastewater. Bioresour Technol 100:74–78
Lee SI, Weon SY, Lee CW, Koopman B (2003) Removal of nitrogen and phosphate from wastewater by addition of bittern. Chemosphere 51:265–271
Marti N, Bouzas A, Seco A, Ferrer J (2008) Struvite precipitation assessment in anaerobic digestion processes. Chem Eng J 141:67–74
Mason RL, Gunst RF, Hess JL (2003) Statistical design and analysis of experiments, eighth applications to engineering and science, 2nd edn. Wiley, New York
Michalowski T, Pietrzyk A (2006) A thermodynamic study of struvite plus water system. Talanta 68:594–601
Moerman W, Carballa M, Vandekerckhove A, Derycke D, Verstraete W (2009) Phosphate removal in agro-industry: pilot- and full-scale operational considerations of struvite crystallization. Water Res 43:1887–1892
Musvoto EV, Wentzel MC, Ekama GA (2000a) Integrated chemical-physical processes modelling—II. Simulating aeration treatment of anaerobic digester supernatants. Water Res 34:1868–1880
Musvoto EV, Wentzel MC, Loewenthal RE, Ekama GA (2000b) Integrated chemical-physical processes modelling—I. Development of a kinetic-based model for mixed weak acid/base systems. Water Res 34:1857–1867
Ohlinger KN, Young TM, Schroeder ED (1998) Predicting struvite formation in digestion. Water Res 32:3607–3614
Scott WD, Wrigley TJ, Webb KM (1991) A computer-model of struvite solution chemistry. Talanta 38:889–895
Song YH, Yuan P, Zheng BH, Peng HF, Yuan F, Gao Y (2007) Nutrients removal and recovery by crystallization of magnesium ammonium phosphate from synthetic swine wastewater. Chemosphere 69:319–324
Stratful I, Scrimshaw MD, Lester JN (2001) Conditions influencing the precipitation of magnesium ammonium phosphate. Water Res 35:4191–4199
Tong J, Chen YG (2009) Recovery of nitrogen and phosphorus from alkaline fermentation liquid of waste activated sludge and application of the fermentation liquid to promote biological municipal wastewater treatment. Water Res 43:2969–2976
Tunay O, Kabdash I, Orhon D, Kolcak S (1997) Ammonia removal by magnesium ammonium phosphate precipitation in industrial wastewater. Water Sci Technol 36:225–228
Uludag-Demirer S (2008) A study on nutrient removal from municipal wastewater by struvite formation using Taguchi's design of experiments. Environ Eng Sci 25:1–10
Uludag-Demirer S, Othman M (2009) Removal of ammonium and phosphate from the supernatant of anaerobically digested waste activated sludge by chemical precipitation. Bioresour Technol 100:3236–3244
Uludag-Demirer S, Demirer GN, Chen S (2005) Ammonia removal from anaerobically digested dairy manure by struvite precipitation. Process Biochem 40:3667–3674
US Environmental Protection Agency (1999) INTEQA2/PRODEFA2. A geochemical assessment model for environmental systems/User manual supplement for version 4.0
Wang JS, Song YH, Yuan P, Peng JF, Fan MH (2006) Modeling the crystallization of magnesium ammonium phosphate for phosphorus recovery. Chemosphere 65:1182–1187
Wang CC, Hao XD, Guo GS, van Loosdrecht MCM (2010) Formation of pure struvite at neutral pH by electrochemical deposition. Chem Eng J 159:280–283
Wilsenach JA, Schuurbiers CAH, van Loosdrecht MCM (2007) Phosphate and potassium recovery from source separated urine through struvite precipitation. Water Res 41:458–466
Wrigley TJ, Scott WD, Webb KM (1992) An improved computer-model of struvite solution chemistry. Talanta 39:1597–1603
Wu Y, Zhou S, Chen D, Zhao R, Li H, Lin Y (2011a) Transformation of metals speciation in a combined landfill leachate treatment. Sci Total Environ 409:1613–1620
Wu Y, Zhou S, Zheng K, Ye X, Qin F (2011b) Mathematical model analysis of Fenton oxidation of landfill leachate. Waste Manag 31:468–474
Ye ZL, Chen SH, Wang SM, Lin LF, Yan YJ, Zhang ZJ et al (2010) Phosphorus recovery from synthetic swine wastewater by chemical precipitation using response surface methodology. J Hazard Mater 176:1083–1088
Zhang C, Chen YG (2009) Simultaneous nitrogen and phosphorus recovery from sludge-fermentation liquid mixture and application of the fermentation liquid to enhance municipal wastewater biological nutrient removal. Environ Sci Technol 43:6164–6170
Acknowledgments
The authors wish to gratefully acknowledge the financial supports from the Key Science and Technology Research Project of People's Republic of China (Grant no. 2008BAE64B05, 2006BAJ04A12-4), the State Key Laboratory of Subtropical Building Science (2009ZB05, 2010ZB04, 2011ZB08), the Department of Science and Technology of Guangdong Province (2007A032500005) for this study, and the Department of Guangzhou Science and Technology Bureau (Grant no. 2008A1-D0011). Additionally, the authors would like to express their sincere appreciation to the anonymous reviewers for their helpful comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Zhihong Xu
Rights and permissions
About this article
Cite this article
Zhou, S., Wu, Y. Improving the prediction of ammonium nitrogen removal through struvite precipitation. Environ Sci Pollut Res 19, 347–360 (2012). https://doi.org/10.1007/s11356-011-0520-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-011-0520-6