Abstract
Purpose and aim
The present study provides an optimization of electrocoagulation process for the simultaneous removal of heavy metals such as mercury, lead, and nickel from water. In doing so, the thermodynamic, adsorption isotherm and kinetic studies were also carried out.
Materials and methods
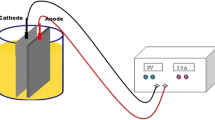
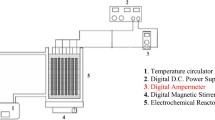
Magnesium alloy, magnesium, aluminum, and mild steel sheet of size 2 dm2 were used as anode and galvanized iron as cathode. To optimize the maximum removal efficiency, different parameters like effect of initial concentration, effect of temperature, pH, and effect of current density were studied. Mercury-, lead-, and nickel-adsorbed magnesium hydroxide coagulant was characterized by SEM and EDAX.
Results
The results showed that the maximum removal efficiency was achieved for mercury, lead, and nickel with magnesium alloy as anode and galvanized iron as cathode at a current density of 0.15 Å/dm2 and pH of 7.0. The adsorption of mercury, lead, and nickel are preferably fitting the Langmuir adsorption isotherm suggests monolayer coverage of adsorbed molecules. The adsorption process follows second-order kinetics. Temperature studies showed that adsorption was endothermic and spontaneous in nature.
Conclusions
The magnesium hydroxide generated in the cell removes the heavy metals present in the water and reduces to a permissible level, making it drinkable.








Similar content being viewed by others
References
Alinsafi A, Khemis M, Pons MN, Leclerc JP, Yaacoubi A, Benhammou A, Nejmeddine A (2005) Electrocoagulation of reactive textile dyes and textile wastewater. Chem Eng Proc 44:461–470
Carrington CD, Montwill B, Bolger PM (2004) An intervention analysis for reduction of exposure to methylmercury from the consumption of sea food by women of child-bearing age. Regul Toxicol Pharma 40:272–280
Casqueira RG, Toren ML, Kohler HM (2006) The removal of zinc from liquid streams by electroflotation. Miner Eng 19:1388–1392
Chen G (2004) Electrochemical technologies in wastewater treatment. Sep Purif Technol 38:11–41
Collado-Sanchez C, Pérez-Pena J, Gelado-Caballero MD, Herrera-Melian JA, Hernandez-Brito JJ (1996) Rapid determination of copper, lead and cadmium in unpurged seawater by adsorptive stripping voltammetry. Anal Chim Acta 320:19–30
Fitzgerald WF, Lamborg CH (2004) Geochemistry of mercury in the environment. In: Holland HD, Turckian KK (eds) Treatise on Geochemistry. Elsevier, Oxford, UK
Freundlich H (1907) Ueber die adsorption in Loesungen. Physic Chem 57:385–470
Golder AK, Samantha AN, Ray S (2006) Removal of phosphate from aqueous solution using calcined metal hydroxides sludge waste generated from electrocoagulation. Sep Purif Technol 52:102–109
Gupta VK (1998) Equilibrium uptake, sorption dynamics, process development and column operations for the removal of copper and nickel from aqueous solution and wastewater using activated slag—a low cost adsorbent. Ind Eng Chem Res 37:192–202
Gupta VK, Ali I (2000) Utilization of bagasse fly ash (a sugar industry waste) for the removal of copper and zinc from wastewater. Sep Purif Technol 18:131–140
Gupta VK, Rastogi A (2008a) Biosorption of lead from aqueous solutions by green algae Spirogyra species: Equilibrium and adsorption kinetics. J Hazard Mater 152:407–414
Gupta VK, Rastogi A (2008b) Equilibrium and kinetic modeling of cadmium (II) biosorption by nonliving algal biomass Oedogonium sp. from aqueous phase. J Hazar Mater 153:759–766
Gupta VK, Rastogi A (2009) Biosorption of hexavalent chromium by raw and acid-treated green alga Oedogonium hatei from aqueous solutions. J Hazard Mater 163:396–402
Gupta VK, Sharma S (2002) Removal of cadmium and zinc from aqueous solutions using red mud. Environ Sci Technol 36:3612–3617
Gupta VK, Sharma S (2003) Removal of zinc from aqueous solutions using bagasse fly ash—a low cost adsorbent. Ind Eng Chem Res 42:6619–6624
Gupta VK, Ali I, Saini VK (1997a) Adsorption studies on the removal of Vertigo Blue 49 and Orange DNA13 from aqueous solutions using carbon slurry developed from a waste material. J Colloid Interface Sci 315:87–93
Gupta VK, Rastogi A, Dwivedi MK, Mohan D (1997b) Process development for the removal of zinc and cadmium from wastewater using slag—a blast furnace waste material. Sep Sci Technol 32:2883–2912
Gupta VK, Srivastava SK, Mohan D, Sharma S (1998) Design parameters for fixed bed reactors of activated carbon developed from fertilizer waste material for the removal of some heavy metal ions. Waste Manage 17:517–522
Gupta VK, Mohan D, Sharma S, Park KT (1999) Removal of chromium (VI) from electroplating industry wastewater using bagasse fly ash—a sugar industry waste material. Environmentalist 19:129–136
Gupta VK, Gupta M, Sharma S (2001) Process development for the removal of lead and chromium from aqueous solutions using red mud—an aluminum industry waste. Water Res 35:1125–1134
Gupta VK, Mohan D, Sharma S (2003a) Removal of lead from wastewater using bagasse fly ash—a sugar industry waste material. Sep Sci Technol 33:1331–1343
Gupta VK, Jain CK, Ali I, Sharma M, Saini VK (2003b) Removal of cadmium and nickel from wastewater using bagasse fly ash—a sugar industry waste. Water Res 37:4038–4044
Gupta VK, Mittal A, Gajbe V, Mittal J (2006a) Removal and recovery of the hazardous azo dye Acid Orange 7 through adsorption over waste materials: bottom ash and de-oiled soya. Ind Eng Chem Res 45:1446–1453
Gupta VK, Mittal A, Kurup L, Mittal J (2006b) Adsorption of a hazardous dye—erythrosine over hen feathers. J Colloid Interface Sci 304:52–57
Gupta VK, Jain R, Mittal A, Mathur M, Shalini S (2007a) Photochemical degradation of the hazardous dye Safranin-T using TiO2 catalyst. J Colloid Interface Sci 309:464–469
Gupta VK, Jain R, Varshney S (2007b) Removal of Reactofix golden yellow 3RFN from aqueous solution using wheat husk- an agricultural waste. J Hazard Mater 142:443–448
Ho YS, McKay G (1998) Sorption of dye from aqueous solution by peat. J Chem Eng 70:115–124
Kurniawan TM, Chan GYS, Lo WH, Babel S (2006) Physico-chemical treatment techniques of wastewater laden with heavy metals. Chem Eng J 118:83–98
Langmuir I (1918) Adsorption of gases on plain surfaces of glass mica platinum. J Am Chem Soc 40:1316–1403
Mckay G, Blair HS, Gardener JR (1982) Adsorption of dyes on Chitin I. Equilibrium studies. J Appl Poly Sci 27:3043–3057
Mercier L, Pinnavaia TJ (1998) A functionalized porous clays heterostructure for heavy metal ion (Hg2+) trapping. Microporous Mesoporous Mater 20:101–106
Mittal A, Jain R, Mittal J, Varshney S, Sirkarwar S (2010) Removal of Yellow ME 7 GL from industrial effluent using electrochemical and sorption technique. Int J Environ Pollusion 43:308–323
Mohan D, Gupta VK, Srivastava SK, Chander S (2001) Kinetics of mercury adsorption from wastewater using activated carbon derived from fertilizer waste. Colloids and Surfaces A 177:169–181
Sharma A, Bhattacharyya KG (2004) Adsorption of chromium(VI) on Azadirachta indica (Neem) leaf powder. Adsorption 10:327–338
Srivastava SK, Gupta VK, Mohan D (1996) Kinetic parameters for the removal of lead and chromium from wastewater using activated Carbon developed from fertilizer waste material. Environ Modell Assessment 1:281–290
Srivastava SK, Gupta VK, Mohan D (1997) Removal of lead and chromium by activated slag—a blast-furnace waste. J Envorn Engg 123:461–468
Tchinda AJ, Ngameni E, Walcarius A (2006) Thiol functionalized clay heterostructures (PCHs) deposited as thin films on carbon electrode: towards mercury(II)sensing. Sens Actuator B121:113–123
Tonle IK, Ngameni E, Walcarius A (2004) From clay- to organoclay-film modified electrodes: tuning charge selectivity in ion exchange voltammetry. Electrochim Acta 49:3435–3443
Ullruich SM, Tanton TW, Abdrasthistova SA (2001) Mercury in the aquatic environment: a review of factors affecting methylation. Crit Rev Environ Sci Technol 31:241–293
Vasudevan S, Lakshmi J (2011) Effects of alternating and direct current in electrocoagulation process on the removal of cadmium from water. Sep Purifi Technol 80:643–651
Vasudevan S, Sozhan G, Ravichandran S, Jayaraj J, Lakshmi J, Margrat Sheela S (2008) Studies on the removal of phosphate from drinking water by electrocoagulation process. Ind Eng Chem Res 47:2018–2023
Vasudevan S, Lakshmi J, Sozhan G (2009) Studies on the removal of iron from drinking water by Electrocoagulation— a Clean Process. Clean 37:45–51
Vasudevan S, Lakshmi J, Vanathi R (2010) Electrochemical coagulation for chromium removal: Process optimization, Kinetics, Isotherm and Sludge characterization. Clean 38:9–16
Veli S, Ozturk T (2005) Kinetic modelling of adsorption of reactive azo dye on powdered activated carbon and pumice. Fresenius Environ Bull 14:347–353
Walcarius A, Etienne M, Sayen S, Lebeau B (2003) Grafted silicas in electroanalysis amorphous versus ordered mesoporous materials. Electroanalysis 15:414–421
WHO Guidelines for drinking water quality (1993) Geneva: World Health Organization. Vol. 1–3:45
Zhu X, Alexandratos SD (2006) Determination of trace levels of mercury in aqueous solutions by inductively coupled plasma atomic emission spectrometry: elimination of the memory effect. Microchem J 81:37–41
Acknowledgment
The authors wish to express their gratitude to the Director, Central Electrochemical Research Institute, Karaikudi, for aid in publishing this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Vinod Kumar Gupta
Rights and permissions
About this article
Cite this article
Vasudevan, S., Lakshmi, J. & Sozhan, G. Optimization of electrocoagulation process for the simultaneous removal of mercury, lead, and nickel from contaminated water. Environ Sci Pollut Res 19, 2734–2744 (2012). https://doi.org/10.1007/s11356-012-0773-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-012-0773-8