Abstract
Purpose
Heavy metals are toxic pollutants released into the environment as a result of different industrial activities. Biosorption of heavy metals from aqueous solutions is a new technology for the treatment of industrial wastewater. The aim of the present research is to highlight the basic biosorption theory to heavy metal removal.
Materials and methods
Heterogeneous cultures mostly dried anaerobic bacteria, yeast (fungi), and protozoa were used as low-cost material to remove metallic cations Pb(II), Cr(III), and Cd(II) from synthetic wastewater. Competitive biosorption of these metals was studied.
Results
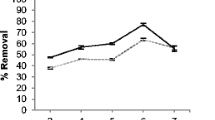
The main biosorption mechanisms were complexation and physical adsorption onto natural active functional groups. It is observed that biosorption of these metals was a surface process. The main functional groups involved in these processes were hydroxyl (–OH) and carboxylic groups (C=O) with 37, 52, and 31 and 21, 14, and 34 % removal of Pb(II), Cr(III), and Cd(II), respectively. Langmuir was the best model for a single system. While extended Langmuir was the best model for binary and ternary metal systems. The maximum uptake capacities were 54.92, 34.78, and 29.99 mg/g and pore diffusion coefficients were 7.23, 3.15, and 2.76 × 10−11 m2/s for Pb(II), Cr(III), and Cd(II), respectively. Optimum pH was found to be 4. Pseudo-second-order was the best model to predict the kinetic process. Biosorption process was exothermic and physical in nature.
Conclusions
Pb(II) offers the strongest component that is able to displace Cr(III) and Cd(II) from their sites, while Cd(II) ions are the weakest adsorbed component.









Similar content being viewed by others
References
APHA (1995) Standard methods for the examination of water and wastewater, 19th edn. American Public Health Association, Washington, DC
Gupta VK, Ali I (2004) Removal of lead and chromium from wastewater using bagasse fly ash—a sugar industry waste. J Colloid Interface Sci 271:321–328
Gupta VK, Ali I (2007) Adsorption studies on the removal of Vertigo Blue 49 and Orange DNA13 from aqueous solutions using carbon slurry developed from a waste material. J Colloid Interface Sci 315(1):87–93
Gupta VK, Rastogi A (2008a) Biosorption of lead from aqueous solutions by green algae Spirogyra species: equilibrium and adsorption kinetics. J Hazard Mater 152(1):407–414
Gupta VK, Rastogi A (2008b) Equilibrium and kinetic modeling of cadmium (II) biosorption by nonliving algal biomass Oedogonium sp. from aqueous phase. J Hazard Mater 153(1-2):759–766
Gupta VK, Rastogi A (2009) Biosoprtion of hexavalent chromium by raw and acid-treated green alga Oedogonium hatei from aqueous solutions. J Hazard Mater 163:396–402
Gupta VK, Rastogi A, Saini VK, Jain N (2006) Biosorption of Cu(II) from aqueous solution by Spirogyra species. J Colloid Interface Sci 296:59–63
Gupta VK, Jain R, Varshney Sh (2007) Removal of reactofix golden yellow 3 RFN from aqueous solution using wheat husk—an agricultural waste. J Hazard Mater 142:443–448
Gupta VK, Rastogi A, Nayak A (2010) Adsorption studies on the removal of hexavalent chromium from aqueous solution using a low cost fertilizer industry waste material. J Colloid Interface Sci 342:135–141
Gupta VK, Agarwal S, Saleh TA (2011) Synthesis and characterization of alumina-coated carbon nanotubes and their application for lead removal. J Hazard Mater 185:17–23
Hawari HA, Mulligan NC (2006) Biosorption of lead (II), copper (II), and nickel (II) by anaerobic granular biomass. Bioresour Technol 97:692–700
Ho YS (2004) Citation review of Lagergren kinetic rate equation on adsorption reactions. Scientometrics 59(1):171–177
Holan ZR, Volesky B, Prasetyo I (1993) Biosorption of cadmium by biomass of marine algae. Biotechnol Bioeng 41:819–825
Huifen L, YanbingL WG, Jiali C, Lin X, Junkang G (2010) Biosorption of Zn(II) by live and dead cells of Streptomyces ciscaucasicus strain CCNWHX 72-14. J Hazard Mater 179:151–159
Jianlong W, Can C (2009) Biosorbents for heavy metals removal and their future. Biotechnol Adv 27:195–226
Lawrence K, Wang JT, Stephen TT, Yung-Tse H (2010) Handbook of environmental engineering, environmental bioengineering. Springer, New York
Lodeiro P, Barriada JL, Herrero R, Sastre de Vicente ME (2006) The marine macroalga Cystoseira baccata as biosorbent for cadmium (II) and lead (II) removal: kinetic and equilibrium studies. Environ Pollut 142:264–273
Naddafi K, Nabizadeh R, Saeedi R, Mahvi AH, Vaezi F, Yaghmaei-ian K, Ghasri A, Nazmara S (2007) Biosorption of lead(II) and cadmium(II) by protonated Sargassum glaucescens biomass in a continuous packed bed column. J Hazard Mater 147:785–791
Niu H, Xu X, Wang J (1993) Removal of lead from aqueous solutions by Penicillium biomass. Biotechnol Bioeng 42:785–807
Ozdemir G, Baysal S (2004) Chromium and aluminum biosorption on Chryseomonas luteola TEM05. Appl Microbiol Biotechnol 64:599–603
Özer A (2003) Comparative study of the biosorption of Pb(II), Ni(II) and Cr(VI) ions onto S. cerevisiae: determination of biosorption heats. J Hazard Mater 100:219–229
Quintelas C, Fernandes B, Castro J, Figueiredo H, Tavares T (2008) Biosorption of Cr(VI) by three different bacterial species supported on granular activated carbon—a comparative study. J Hazard Mater 153:799–809
Reddad Z, Gerente C, Andres Y, Le C (2002) Adsorption of several metal ions onto a low-cost biosorbent: kinetic and equilibrium studies. Environ Sci Technol 36(9):2067–2073
Sulaymon AH, Ahmed KW (2008) Competitive adsorption of furfural and phenolic compounds onto activated carbon in fixed bed column. Environ Sci Technol 42(2):392–397
Sulaymon AH, Ebrahim SE (2010) Removal of lead, cadmium, and mercury ions using biosorption. Desalin Water Treat 24:344–352
Sulaymon HA, Abidb AB, Al-Najar AJ (2009) Removal of lead copper chromium and cobalt ions onto granular activated carbon in batch and fixed-bed adsorbers. Chem Eng J 155(3):647–653
Tuzun I, Bayramoglu G, Alcin YE, Basaran G, Celik G, Arica MY (2005) Equilibrium and kinetic studies on biosorption of Hg(II), Cd(II) and Pb(II) ions onto microalgae Chlamydomonas reinhardtii. J Environ Manag 77:85–92
Vijayaraghavan K, Yun Y-S (2008) Bacterial biosorbents and biosorption. Biotechnol Adv 26:266–291
Volesky B, May H, Holan ZR (2004) Cadmium biosorption by Saccharomyces cerevisiae. Biotechnol Bioeng 41:826–829
Volker K (1999) Simulation of liquid chromatography and simulated moving bed system. Technical report by Hamburg-Hamburg University (TUHH)
Ziagova M, Dimitriadis G, Aslanidou D, Papaioannou X, Litopoulou TE, Liakopoulou KM (2007) Comparative study of Cd(II) and Cr(VI) biosorption on Staphylococcus xylosus and Pseudomonas sp. in single and binary mixtures. Bioresour Technol 98(15):2859–2865
Zubeyde B, Ercan C, Mehmet D (2009) Equilibrium and thermodynamic studies on biosorption of Pb(II) onto Candida albicans biomass. J Hazard Mater 161:62–67
Acknowledgments
We would like to express our sincere thanks and deep gratitude to the Ministry of Oil, Petroleum Development and Research Center, Baghdad, Iraq for supporting this work financially.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Vinod Kumar Gupta
Rights and permissions
About this article
Cite this article
Sulaymon, A.H., Ebrahim, S.E. & Mohammed-Ridha, M.J. Equilibrium, kinetic, and thermodynamic biosorption of Pb(II), Cr(III), and Cd(II) ions by dead anaerobic biomass from synthetic wastewater. Environ Sci Pollut Res 20, 175–187 (2013). https://doi.org/10.1007/s11356-012-0854-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-012-0854-8