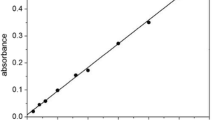
Abstract
Purpose and aim
In general, direct current (DC) is used in an electrocoagulation processes. In this case, an impermeable oxide layer may form on the cathode as well as corrosion formation on the anode due to oxidation. This prevents the effective current transfer between the anode and cathode, so the efficiency of electrocoagulation processes declines. These disadvantages of DC have been diminished by adopting alternating current (AC) in electrocoagulation processes. The main objective of this study is to investigate the effects of AC and DC on the removal of copper from water using magnesium alloy as anode and cathode.
Materials and methods
Magnesium alloy of size 2.0 dm2 was used as anode and as cathode. To optimize the maximum removal efficiency, different parameters like effect of initial concentration, effect of temperature, pH, and effect of current density were studied. Copper adsorbed magnesium hydroxide coagulant was characterized by SEM, EDAX, XRD, and FTIR.
Results
The results showed that the optimum removal efficiency of copper is 97.8 and 97.2 % with an energy consumption of 0.634 and 0.996 kWh/m3 at a current density of 0.025 A/dm2, pH of 7.0 for AC and DC, respectively. The adsorption of copper is preferably fitting the Langmuir adsorption isotherm for both AC and DC respectively. The adsorption process follows the second-order kinetics model with good correlation. Temperature studies showed that adsorption was endothermic and spontaneous in nature.
Conclusions
The magnesium hydroxide generated in the cell removes the copper present in the water, reducing the copper concentration to less than 1 mg/L, making it safe for drinking. The results of the scale-up study show that the process was technologically feasible.












Similar content being viewed by others
References
Adhoum N, Monser L (2004) Decolourization and removal of phenolic compounds from olive mill wastewater by electrocoagulation. Chem Eng Process 43:1281–1287
Ajmal M, Khan A, Ahmad S, Ahmad A (1998) Role of sawdust in the removal of copper(II) from industrial wastes. Water Res 32:3085–3091
Ali I (2010) The quest for active carbon adsorbent substitutes: inexpensive adsorbents for toxic metal ions removal from wastewater. Sep Purf Rev 39:95–171
Ali I, Gupta VK (2007) Advances in water treatment by adsorption technology. Nat Protoc 1:2661–2667
Ali I, Khan TA, Asim M (2011) Removal of arsenic from water by electrocoagulation and electrodialysis techniques. Sepn Purfn Rev 40:25–42
Ali I, Khan TA, Asim M (2012) Removal of arsenate from ground water by electro-coagulation method. Environ Sci Polln Res. doi:10.1007/s11356-011-0681-3
Allen SJ, Mckay G, Khader KHY (1989) Intraparticle diffusion of basic dye during adsorption onto Sphagnum peat. Environ Pollut 56:39–50
Bailey SE, Olin TJ, Bricka RM, Adrian DD (1999) A review of potentially low-cost sorbents for heavy metals. Water Res 33:2469–2479
Benaissa H, Elouchd MA (2007) Removal of copper ions from aqueous solutions by dried sunflower leaves. Chem Eng Process 46:614–622
Billon L, Meric V, Castetbon A, Francois J, Desbrieres J (2006) Removal of copper ions from water of boilers by a modified natural based corncobs. J Appl Polym Sci 102:4637–4645
Boujelben N, Bouzid J, Elouear Z (2009) Adsorption of nickel and copper onto natural iron oxide-coated sand from aqueous solutions: study in single and binary systems. J Hazard Mater 163:376–382
Bouzid J, Elouear Z, Ksibi M, Feki M, Montiel A (2008) A study on removal characteristics of copper from aqueous solution by sewage sludge and pomace ashes. J Hazard Mater 152:838–845
Carlesi Jara C, Fino D, Specchia V, Saracco G, Spinelli P (2007) Electrochemical removal of antibiotics from wastewaters. Appl CatalsB: Environ 70:479–487
Carlos A, Huitle M, Ferro S (2006) Electrochemical oxidation of organic pollutants for the wastewater treatment: direct and indirect processes. Chem Soc Rev 35:1324–1340
Chen G (2004) Electrochemical technologies in wastewater treatment. Sep Purif Technol 38:11–41
Christensen PA, Egerton TA, Lin WF, Meynet P, Shaoa ZG, Wright NG (2006) A novel electrochemical device for the disinfection of fluids by OH radicals. Chem Commun 38:4022–4023
CPCB,Central Pollution Control Board (2002) Government of India, Delhi
Demiral H, Demiral I, Tumsek F, Karacbacakoglu B (2008) Adsorption of chromium (Vl) from aqueous solution by activated solution by activated carbon derived from olive bagasse and applicability of different adsorption models. Chem Eng J 144:188–195
Fiol N, Villaescesa I, Martinez M, Miralles N, Poch J, Serarols J (2006) Sorption of Pb(II), Ni(II), Cu(II) and Cd(II) from aqueous solutions by olive stone waste. Sep Purif Technol 50:132–140
Gardea-Torresde JL, Tang L, Salvador JM (1996) Copper adsorption by esterified and unesterified fractions of Sphagnum peat moss and its different humic substances. J Hazard Mater 48:191–206
Golder AK, Samantha AN, Ray S (2006) Removal of phosphate from aqueous solution using calcined metal hydroxides sludge waste generated from electrocoagulation. Sep Purif Technol 52:102–109
Goyal RN, Gupta VK, Sangal A, Bachheti N (2005) Voltammetric determination of uric acid at a fullerene-C60-modified glassy carbon electrode. Electroanalysis 17:2217–2223
Goyal RN, Gupta VK, Oyama M, Bachheti N (2007a) Gold nanoparticles modified indium tin oxide electrode for the simultaneous determination of dopamine and serotonin: application in pharmaceutical formulations and biological fluids. Talanta 72:976–983
Goyal RN, Gupta VK, Bachheti N (2007b) Voltammetric determination of adenosine and guanosine using fullerene-C60-modified glassy carbon electrode. Talanta 71:1110–1117
Gupta VK (1998) Equilibrium uptake, sorption dynamics, process development and column operations for the removal of copper and nickel from aqueous solution and wastewater using activated slag-a low cost adsorbent. Ind Eng Chem Res 37:192–202
Gupta VK, Ali I (2000) Utilization of bagasse fly ash (a sugar industry waste) for the removal of copper and zinc from wastewater. Sep Purif Technol 18:131–140
Gupta VK, Rastogi A (2008a) Biosorption of lead from aqueous solutions by green algae Spirogyra species: equilibrium and adsorption kinetics. J Hazard Mater 152:407–414
Gupta VK, Rastogi A (2008b) Equilibrium and kinetic modeling of cadmium (II) biosorption by nonliving algal biomass Oedogonium sp. from aqueous phase. J Hazard Mater 153:759–766
Gupta VK, Rastogi A (2009) Biosorption of hexavalent chromium by raw and acid-treated green alga Oedogonium hatei from aqueous solutions. J Hazard Mater 163:396–402
Gupta VK, Rastogi A (2010) Adsorption studies on the removal of hexavalent chromium from aqueous solution using a low cost fertilizer industry waste material. J Colloid Interface Sci 342:135–141
Gupta VK, Sharma S (2002) Removal of cadmium and zinc from aqueous solutions using red mud. Environ Sci Technol 36:3612–3617
Gupta VK, Ali I, Saini VK (1997a) Adsorption studies on the removal of Vertigo Blue 49 and Orange DNA13 from aqueous solutions using carbon slurry developed from a waste material. J Colloid Interface Sci 315:87–93
Gupta VK, Rastogi A, Dwivedi MK, Mohan D (1997b) Process development for the removal of zinc and cadmium from wastewater using slag—a blast furnace waste material. Sep Sci Technol 32:2883–2912
Gupta VK, Srivastava SK, Mohan D, Sharma S (1998) Design parameters for fixed bed reactors of activated carbon developed from fertilizer waste material for the removal of some heavy metal ions. Waste Manage 17:517–522
Gupta VK, Mohan D, Sharma S, Park KT (1999) Removal of chromium (VI) from electroplating industry wastewater using bagasse fly ash—a sugar industry waste material. Environmentalist 19:129–136
Gupta VK, Gupta M, Sharma S (2001) Process development for the removal of lead and chromium from aqueous solutions using red mud-an aluminum industry waste. Water Res 35:1125–1134
Gupta VK, Mangla R, Agarwal S (2002) Pb (II) selective potentiometric sensor based on 4-tert-Butylcalix [4] arene in PVC matrix. Electroanalysis 14:1127–1132
Gupta VK, Mohan D, Sharma S (2003a) Removal of lead from wastewater using bagasse fly ash—a sugar industry waste material. Sep Sci Technol 33:1331–1343
Gupta VK, Jain CK, Ali I, Sharma M, Saini VK (2003b) Removal of cadmium and nickel from wastewater using bagasse fly ash—a sugar industry waste. Water Res 37:4038–4044
Gupta VK, Mittal A, Gajbe V, Mittal J (2006a) Removal and recovery of the hazardous azo dye acid orange 7 through adsorption over waste materials: bottom ash and de-oiled soya. Ind Eng Chem Res 45:1446–1453
Gupta VK, Mittal A, Kurup L, Mittal J (2006b) Adsorption of a hazardous dye—erythrosine over hen feathers. J Colloid Interface Sci 304:52–57
Gupta VK, Jain AK, Kumar P, Agarwal S, Maheshwari G (2006c) Chromium(III)-selective sensor based on tri-o-thymotide in PVC matrix. Sens Actuat B: Chem 113:182–186
Gupta VK, Jain R, Mittal A, Mathur M, Shalini S (2007a) Photochemical degradation of the hazardous dye Safranin-T using TiO2 catalyst. J Colloid Interface Sci 309:464–469
Gupta VK, Jain R, Varshney S (2007b) Removal of Reactofix golden yellow 3RFN from aqueous solution using wheat husk- an agricultural waste. J Hazard Mater 142:443–448
Gupta VK, Mittal A, Jain R, Mathur M, Sikarwar S (2007c) Photochemical degradation of hazardous dye—Safaranin-T Using TiO2 Catalyst. J Colloid Interface Sci 309:460–465
Gupta VK, Singh AK, Gupta B (2007d) Schiff bases as cadmium (II) selective ionophores in polymeric membrane electrodes. Anal Chim Acta 583:340–348
Gupta VK, Jain R, Varshney S (2007e) Electrochemical removal of hazardous dye Reactofix Red 3 BFN from industrial effluents. J Colloid Interface Sci 312:292–296
Gupta VK, Ali I, Saini VK (2007f) Defluoridation of wastewaters using waste carbon slurry. Water Res 41:3307–3316
Gupta VK, Goyal RN, Sharma RA (2009a) Novel PVC membrane based alizarin sensor and its application; determination of vanadium, zirconium and molybdenum. Int J Electrochem Sci 4:156–172
Gupta VK, Al Khayat M, Singh AK, Pal MK (2009b) Nano level detection of Cd (II) using poly (vinyl chloride) based membranes of Schiff bases. Anal Chim Acta 634:36–43
Gupta VK, Goyal RN, Sharma RA (2009c) Comparative studies of neodymium (III)-selective PVC membrane sensors. Analytica Chim Acta 647:66–71
Gupta VK, Carrott PJM, Ribeiro Carrott MML (2009d) Low cost adsorbents: growing approach to wastewater treatment—a review. Critical Rev Environ Sci Technol 39:783–842
Ho YS, McKay G (1998) A comparison of chemisorption kinetic models applied to pollutant removal on various sorbents. Process Saf Environ Prot 76:332–340
Jain AK, Gupta VK, Singh LP (1995a) Neutral carrier and organic resin based membranes as sensors for uranyl ions. Anal Proc Anal Commun (RSC) 32:263–265
Jain AK, Gupta VK, Sahoo BB, Singh LP (1995b) Copper (II)-selective electrodes based on macrocyclic compounds. Anal Proc Anal Commun(RSC) 32:99–101
Jain AK, Gupta VK, Khurana U, Singh LP (1997) A new membrane sensor for UO2+, based on 2-hydroxyacetophenoneoxime—thioureatrioxane resin. Electroanalysis 9:857–860
James R, Sampath K, Selvamani P (2006) Effect of ion-exchanging agent, zeolite on removal of copper in water and improvement of growth in Oreochromis mossambicus (Peters). Asian Fisheries Sci 13:317–325
Larous S, Meniai H, Bencheikh Lehocine M (2005) Experimental study of the removal of copper from aqueous solutions by adsorption using sawdust. Desalination 185:483–490
Leea C, Yanga W, Hsiehb C (2004) Removal of copper (II) by manganese-coated sandin a liquid fluidized-bed reactor. J Hazard Mater B114:45–51
Li X, Ma GB, Liu YY (2009) Synthesis and characterization of magnesium hydroxide using a bubbling setup. Ind Eng Chem Res 48:763–768
Mittal A, Jain R, Mittal J, Varshney S, Sirkarwar S (2010) Removal of Yellow ME 7 GL from industrial effluent using electrochemical and sorption technique. Int J Environ Pollution 43:308–323
Miwa DW, Malpass GRP, Machado SAS, Motheo AJ (2006) Electrochemical degradation of carbaryl on oxide electrodes. Water Res 40:281–289
Mohan D, Gupta VK, Srivastava SK, Chander S (2001) Kinetics of mercury adsorption from wastewater using activated carbon derived from fertilizer waste. Colloids and Surfaces A 177:169–181
Mrozowski J, Zielinski J (1983) Studies of zinc and lead removal from industrial wastes by electrocoagulation. Environ Prot Eng 9:77–85
Oke IA, Olarinoye NO, Adewusi SRA (2008) Adsorption kinetics for arsenic removal from aqueous solutions by untreated powdered eggshell. Adsorption 14:73–83
Onder E, Koparal AS, Ogutveren UB (2007) An alternative method for the removal of surfactants from water: electrochemical coagulation. Sep Purif Technol 52:527–532
Onmez G, Aksu Z (1999) The effect of copper(II) ions on the growth and bioaccumulation properties of some yeasts. Process Biochem 35:35–142
Ozcan A, Ozcan AS, Tunali S, Akar T, Kiran I (2005) Determination of the equilibrium, kinetic and thermodynamic parameters of adsorption of copper (II) ions onto seeds of Capsicum annuum. J Hazard Mater B124:200–208
Ozer A, Ozer D, Ozer A (2004) The adsorption of copper(II) ions onto dehydrated wheat bran (DWB): determination of the equilibrium and thermodynamic parameters. Process Biochem 39:2183–2191
Papandreou A, Stournaras CJ, Panias D (2007) Copper and cadmium adsorption on pellets made from fired coal fly ash. J Hazard Mater 148:538–547
Prasad MNV, Freitas H (2000) Removal of toxic metals from solution by leaf, stem and root phytomass of Quercus ilex L. (holly oak). Environ Pollut 110:277–283
Prasanna Kumar Y, King P, Prasad VSRK (2006) Removal of copper from aqueous solution using Ulva fasciata sp.—a marine green algae. J Hazard Mater B137:367–373
Saeed A, Iqbal M, Akhtar MW (2005) Removal and recovery of lead(II) from single and multimetal (Cd, Cu, Ni, Zn) solutions by crop milling waste (black gram husk). J Hazard Mater B117:65–73
Sarioglu M, May A, Cebeci Y (2005) Removal of copper from aqueous solutions by phosphate rock. Desalination 181:303–311
Shukla SP, Sakhardane VD (1992) Column studies on metal ion removal by dyed cellulosic materials. J Appl Poly Sci 44:903–910
Srivastava SK, Gupta VK, Dwivedi MK, Jain S (1995) Caesium PVC-Crown (dibenzo-24-crown-8) based membrane sensor. Anal Proc Anal Commun (RSC) 32:21–23
Srivastava SK, Gupta VK, Mohan D (1996a) Kinetic parameters for the removal of lead and chromium from wastewater using activated Carbon developed from fertilizer waste material. Environ Modell Assessment 1:281–290
Srivastava SK, Gupta VK, Jain S (1996b) A PVC-based benzo-15-crown-5 membrane sensor for cadmium. Electroanalysis 8:938–940
Srivastava SK, Gupta VK, Mohan D (1997) Removal of lead and chromium by activated slag—a blast-furnace waste. J Envorn Engg 123:461–468
Tan IAW, Hameed BH, Ahmed AL (2007) Equilibrium and kinetics studies on the basic dye adsorption by palm fibre activated carbon. Chem Eng J 127:111–119
Vasudevan S, Lakshmi J (2011) Effects of alternating and direct current in electrocoagulation process on the removal of cadmium from water. Sep Purifi Technol 80:643–651
Vasudevan S, Sozhan G, Ravichandran S, Jayaraj J, Lakshmi J, Margrat Sheela S (2008) Studies on the removal of phosphate from drinking water by electrocoagulation process. Ind Eng Chem Res 47:2018–2023
Vasudevan S, Lakshmi J, Sozhan G (2009) Studies on the removal of iron from drinking water by electrocoagulation—a clean process. Clean 37:45–51
Vasudevan S, Lakshmi J, Vanathi R (2010a) Electrochemical coagulation for chromium removal: process optimization, kinetics, isotherm and sludge characterization. Clean 38:9–16
Vasudevan S, Margrat Sheel S, Lakshmi J, Sozhan G (2010b) Optimization of the process parameters for the removal of boron from drinking water by electrocoagulation–a clean technology. J Chem Technol Biotech 85:926–933
Vasudevan S, Lakshmi J, Sozhan G (2010c) Studies relating to removal of arsenate by electrochemical coagulation optimization, kinetics, coagulant characterisation. Sep Sci Technol 45:1313–1325
Vasudevan S, Lakshmi J, Sozhan G (2010d) Studies on the removal of arsenate by electrochemical coagulation using aluminium alloy anode. Clean 38:506–515
Vasudevan S, Lakshmi J, Packiyam M (2010e) Electrocoagulation studies on removal of cadmium using magnesium electrode. J Appl Electrochem 40:2023–2032
Villaescesa I, Fiol N, Martinez M, Miralles N, Poch J, Serarols J (2004) Removal of copper and nickel ions from aqueous solutions by grape stalk wastes. Water Res 38:992–1002
Vinikour WS, Goldstein RM, Anderson RV (1980) Bioaccumulation patterns of zinc, copper, cadmium and lead in selected fish species from the Fox River, Illinois. Bull Environ Contam Toxicol 24:727–734
Wan Ngah WS, Hanafiah MAKM (2008) Adsorption of copper on rubber (Hevea brasiliensis) leaf powder: kinetic, equilibrium and thermodynamic studies. Biochemical En J 39:521–530
Weber WJ Jr, Morris JC (1963) Kinetics of adsorption on carbon from solutions. J SanitDiv Am Soc Civ Eng 89:31–59
Wu Z, Joo H, Lee K (2005) Kinetics and thermodynamics of the organic dye adsorption on the mesoporous hybrid xerogel. Chem Eng J 112:227–236
Yang XY, Al-Duri B (2001) Application of branched pore diffusion model in the adsorption of reactive dyes on activated carbon. Chem Eng J 83:15–23
Yu B, Zhang Y, Shukla A, Shukla SS, Dorris KL (2000) The removal of heavy metal from aqueous solutions by sawdust adsorption—removal of copper. J Hazard Mater 80:33–42
Acknowledgment
The authors wish to express their gratitude to the Director, Central Electrochemical Research Institute, Karaikudi, for aid in publishing this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Vinod Kumar Gupta
Rights and permissions
About this article
Cite this article
Kamaraj, R., Ganesan, P., Lakshmi, J. et al. Removal of copper from water by electrocoagulation process—effect of alternating current (AC) and direct current (DC). Environ Sci Pollut Res 20, 399–412 (2013). https://doi.org/10.1007/s11356-012-0855-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-012-0855-7