Abstract
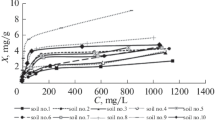
In the present study, the feasibility of soil used as a low-cost adsorbent for the removal of Cu2+, Zn2+, and Pb2+ ions from aqueous solution was investigated. The kinetics for adsorption of the heavy metal ions from aqueous solution by soil was examined under batch mode. The influence of the contact time and initial concentration for the adsorption process at pH of 4.5, under a constant room temperature of 25 ± 1 °C were studied. The adsorption capacity of the three heavy metal ions from aqueous solution was decreased in order of Pb2+ > Cu2+ > Zn2+. The soil was characterized by Fourier transform infrared (FTIR) spectroscopy, scanning electron microscopic-energy dispersive X-ray (SEM-EDX), and Brunauer, Emmett, and Teller (BET) surface area analyzer. From the FTIR analysis, the experimental data was corresponded to the peak changes of the spectra obtained before and after adsorption process. Studies on SEM-EDX showed distinct adsorption of the heavy metal ions and the mineral composition in the study areas were determined to be silica (SiO2), alumina (Al2O3), and iron(III) oxide (FeO3). A distinct decrease of the specific surface area and total pore volumes of the soil after adsorption was found from the BET analysis. The experimental results obtained were analyzed using four adsorption kinetic models, namely pseudo-first-order, pseudo-second-order, Elovich, and intraparticle diffusion. Evaluating the linear correlation coefficients, the kinetic studies showed that pseudo-second-order equation described the data appropriable than others. It was concluded that soil can be used as an effective adsorbent for removing Cu2+, Zn2+, and Pb2+ ions from aqueous solution.












Similar content being viewed by others
References
Das B, Mondal NK, Bhaumik R, Roy P, Pal KC, Das CR (2013) Removal of copper from aqueous solution using alluvial soil of Indian origin: equilibrium, kinetic and thermodynamic study. J Mater Environ Sci 4(4):392–408
Dube A, Zbytniewski R, Kowalkowski T, Cukrowska E, Buszewski B (2000) Adsorption and migration of heavy metals in soil. Pol J Environ Stud 10(1):1–10
Fonseca B, Maio H, Quintelas C, Teixeira A, Tavares T (2009) Retention of Cr(VI) and Pb(II) on a loamy sand soil: kinetics, equilibria and breakthrough. Chem Eng J 152:212–219
Heggy SEM, Komy ZR, Shaker AM, El-Sayed MEA (2013) Kinetics of zinc adsorption on soil minerals in the absence and presence of humic acid. J Am Sci 9(4):523–533
Ho YS, McKay G (1998) A comparison of chemisorption kinetic models applied to pollutant removal on various sorbents. Process Saf Environ Prot 76(4):332–340
Horsfall M, Vicente JL (2007) Kinetic study of liquid-phase adsorptive removal of heavy metal ions by almond tree (Terminalia catappa L.) leaves waste. Bull Chem Soc Ethiop 21(3):349–362
Jodeh S, Abu-Obaid AA (2011) The kinetic study of adsorption of copper metal Ion in selected contaminated red soil samples in Palestine. J Chem Eng Data 5:873–879
McLean JE, Bledsoe BE (1992) Behavior of Metals in Soils. Available from: http://www.epa.gov/superfund/remedytech/tsp/download/issue14.pdf. Accessed on 24 Oct 2013
Merlain TG, Nsami NJ, Mbadcam KJ (2013) Adsorption of copper (II) ions from aqueous solution onto synthetic goethite and two naturally available red soils from Yaoundé –Cameroon. Br Biotechnol J 3(3):221–235
Mohammed SAS (2012) Retention capacity of soils and amended soils for heavy metal ions. Phys Chem News 66:1–16
Oladoja NA, Aboluwoye CO, Oladimeji YB (2008) Kinetics and isotherm studies on methylene blue adsorption onto ground palm kernel coat. Turk J Eng Environ Sci 32:303–312
Orisakwe OE, Nduka JK, Amadi CN, Dike DO, Bede O (2012) Heavy metals health risk assessment for population via consumption of food crops and fruits in Owerri, South Eastern, Nigeria. Chem Cent J 6:77–83
Paliwal V (2006) Study of Lead Sorption on Magnetite at High Temperatures. Available from: http://digital.library.unt.edu/ark:/67531/metadc5445/. Accessed on 9 Apr 2014
Payne KB, Abdel-Fattah TM (2005) Adsorption of arsenate and arsenite by iron-treated activated carbon and zeolites: effects of pH, temperature, and ionic strength. J Environ Sci Health 40:723–749
Shah IK, Pre P, Alappat BJ (2013) Steam regeneration of adsorbents: an experimental and technical review. J Chem Sci 2(4):1078–1088
Shahmohammadi-Kalalagh S, Babazadeh H, Nazemi AH, Manshouri M (2011) Isotherm and kinetic studies on adsorption of Pb, Zn and Cu by kaolinite. Caspian J Environ Sci 9(2):243–255
Shin WS, Kang K, Kim YK (2014) Adsorption characteristics of multi-metal ions by Red Mud, zeolite, limestone, and oyster shell. Environ Eng Res 19(1):15–22
Singh R, Gautam N, Mishra A, Gupta R (2011) Heavy metals and living systems: an overview. Indian J Pharmacol 43(3):246–253
Tilson HA (2013) Disease Outcomes, Exposures, and Methodologies and Populations. Available from: http://ehp.niehs.nih.gov/wp-content/uploads/2013/10/CH_2013_final.pdf. Accessed on 19 Dec 2013
Uzun İ, Güzel F (2000) Adsorption of some heavy metal ions from aqueous solution by activated carbon and comparison of percent adsorption results of activated carbon with those of some other adsorbents. Turk J Chem 24:291–297
Yadav PR, Tyagi R (2006) Crop Biotechnology. Discovery Publishing House, New Delhi
Acknowledgments
The authors would like to thank Universiti Malaysia Sarawak for the use of laboratory facilities and equipments. This research was funded by Fundamental Research Grant Scheme (FRGS/02(17)/738/2010(24)) from the Ministry of Higher Education, Malaysia and CoERE Grant (CoERE/Grant/2013/04).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Zhihong Xu
Rights and permissions
About this article
Cite this article
Lim, SF., Lee, A.Y.W. Kinetic study on removal of heavy metal ions from aqueous solution by using soil. Environ Sci Pollut Res 22, 10144–10158 (2015). https://doi.org/10.1007/s11356-015-4203-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-4203-6