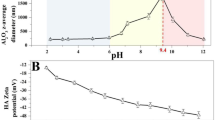
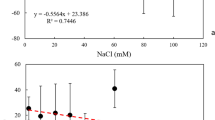
Abstract
The stability of CuO nanoparticles (NPs) is expected to play a key role in the environmental risk assessment of nanotoxicity in aquatic systems. In this study, the effect of alginate (model polysaccharides) on the stability of CuO NPs in various environmentally relevant ionic strength conditions was investigated by using time-resolved dynamic light scattering. Significant aggregation of CuO NPs was observed in the presence of both monovalent and divalent cations. The critical coagulation concentrations (CCC) were 54.5 and 2.9 mM for NaNO3 and Ca(NO3)2, respectively. The presence of alginate slowed nano-CuO aggregation rates over the entire NaNO3 concentration range due to the combined electrostatic and steric effect. High concentrations of Ca2+ (>6 mM) resulted in stronger adsorption of alginate onto CuO NPs; however, enhanced aggregation of CuO NPs occurred simultaneously under the same conditions. Spectroscopic analysis revealed that the bridging interaction of alginate with Ca2+ might be an important mechanism for the enhanced aggregation. Furthermore, significant coagulation of the alginate molecules was observed in solutions of high Ca2+ concentrations, indicating a hetero-aggregation mechanism between the alginate-covered CuO NPs and the unabsorbed alginate. These results suggested a different aggregation mechanism of NPs might co-exist in aqueous systems enriched with natural organic matter, which should be taken into consideration in future studies.

Hetero-aggregation mechanism of CuO nanoparticles and alginate under high concentration of Ca2+





Similar content being viewed by others
References
Adeleye AS, Conway JR, Perez T, Rutten P, Keller AA (2014) Influence of extracellular polymeric substances on the long-term fate, dissolution, and speciation of copper-based nanoparticles. Environ Sci Technol 48:12561–12568
Aiken GR, Hsu-Kim H, Ryan JN (2011) Influence of dissolved organic matter on the environmental fate of metals, nanoparticles, and colloids. Environ Sci Technol 45:3196–3201
Applerot G, Lellouche J, Lipovsky A, Nitzan Y, Lubart R, Gedanken A, Banin E (2012) Understanding the antibacterial mechanism of CuO nanoparticles: revealing the route of induced oxidative stress. Small 8:3326–3337
Aruoja V, Dubourguier HC, Kasemets K, Kahru A (2009) Toxicity of nanoparticles of CuO, ZnO and TiO2 to microalgae Pseudokirchneriella subcapitata. Sci Total Environ 407(4):1461–1468
Baalousha M (2009) Aggregation and disaggregation of iron oxide nanoparticles: influence of particle concentration, pH and natural organic matter. Sci Total Environ 407(6):2093–2101
Burkart C, von Tümpling W, Berendonk T, Jungmann D (2015) Nanoparticles in wastewater treatment plants: a novel acute toxicity test for ciliates and its implementation in risk assessment. Environ Sci Pollut R 22(10):7485–7494
Chang YN, Zhang M, Xia L, Zhang J, Xing G (2012) The toxic effects and mechanisms of CuO and ZnO nanoparticles. Materials 5:2850–2871
Chen KL, Elimelech M (2007) Influence of humic acid on the aggregation kinetics of fullerene (C60) nanoparticles in monovalent and divalent electrolyte solutions. J Colloid Interface Sci 309(1):126–134
Chen KL, Mylon SE, Elimelech M (2006) Aggregation kinetics of alginate-coated hematite nanoparticles in monovalent and divalent electrolytes. Environ Sci Technol 40:1516–1523
Gallego-Urrea JA, Holmberg JP, Hassellöv M (2014) Influence of different types of natural organic matter on titania nanoparticle stability: effects of counter ion concentration and pH. Environ Sci: Nano 1(2):181–189
Grillo R, Rosa AH, Fraceto LF (2015) Engineered nanoparticles and organic matter: a review of the state-of-the-art. Chemosphere 119:608–619
Hou J, Miao L, Wang C, Wang P, Ao Y, Lv B (2015) Effect of CuO nanoparticles on the production and composition of extracellular polymeric substances and physicochemical stability of activated sludge flocs. Bioresour Technol 176:65–70
Huangfu X, Jiang J, Ma J, Liu Y, Yang J (2013) Aggregation kinetics of manganese dioxide colloids in aqueous solution: influence of humic substances and biomacromolecules. Environ Sci Technol 47:10285–10292
Illés E, Tombácz E (2006) The effect of humic acid adsorption on pH-dependent surface charging and aggregation of magnetite nanoparticles. J Colloid Interface Sci 295(1):115–123
Karlsson HL, Cronholm P, Gustafsson J, Moller L (2008) Copper oxide nanoparticles are highly toxic: a comparison between metal oxide nanoparticles and carbon nanotubes. Chem Res Toxicol 21:1726–1732
Khan SS, Mukherjee A, Chandrasekaran N (2011) Impact of exopolysaccharides on the stability of silver nanoparticles in water. Water Res 45(16):5184–5190
Kloster N, Avena M (2015) Interaction of humic acids with soil minerals: adsorption and surface aggregation induced by Ca2+. Environ Chem. http://dx.doi.org/10.1071/EN14157
Kloster N, Brigante M, Zanini G, Avena M (2013) Aggregation kinetics of humic acids in the presence of calcium ions. Colloid Surface A 427:76–82
Kroll A, Behra R, Kaegi R, Sigg L (2014) Extracellular polymeric substances (EPS) of freshwater biofilms stabilize and modify CeO2 and Ag nanoparticles. PLoS One 9, e110709
Li WW, Yu HQ (2014) Insight into the roles of microbial extracellular polymer substances in metal biosorption. Bioresour Technol 160:15–23
Liu X, Wazne M, Han Y, Christodoulatos C, Jasinkiewicz KL (2010) Effects of natural organic matter on aggregation kinetics of boron nanoparticles in monovalent and divalent electrolytes. J Colloid Interface Sci 348(1):101–107
Loosli F, Le Coustumer P, Stoll S (2015) Effect of electrolyte valency, alginate concentration and pH on engineered TiO2 nanoparticle stability in aqueous solution. Sci Total Environ 535:28–34
Majedi SM, Kelly BC, Lee HK (2014) Role of combinatorial environmental factors in the behavior and fate of ZnO nanoparticles in aqueous systems: a multiparametric analysis. J Hazard Mater 264:370–379
Miao L, Wang C, Hou J, Wang P, Ao Y, Dai S, Lv B (2014) Effects of pH and natural organic matter (NOM) on the adsorptive removal of CuO nanoparticles by periphyton. Environ Sci Pollut R 22(10):7696–7704
Nowack B, Bucheli TD (2007) Occurrence, behavior and effects of nanoparticles in the environment. Environ Pollut 150:5–22
Nowack B, Ranville JF, Diamond S, Gallego‐Urrea JA, Metcalfe C, Rose J, Klaine SJ (2012) Potential scenarios for nanomaterial release and subsequent alteration in the environment. Environ Toxicol Chem 31(1):50–59
Philippe A, Schaumann GE (2014) Interactions of dissolved organic matter with natural and engineered inorganic colloids: a review. Environ Sci Technol 48:8946–8962
Shen MH, Yin YG, Booth A, Liu JF (2015) Effects of molecular weight-dependent physicochemical heterogeneity of natural organic matter on the aggregation of fullerene nanoparticles in mono-and di-valent electrolyte solutions. Water Res 71:11–20
Shih YH, Zhuang CM, Tso CP, Lin CH (2012) The effect of electrolytes on the aggregation kinetics of titanium dioxide nanoparticle aggregates. J Nanopart Res 14(8):1–11
Son J, Vavra J, Forbes VE (2015) Effects of water quality parameters on agglomeration and dissolution of copper oxide nanoparticles (CuO-NPs) using a central composite circumscribed design. Sci Total Environ 521:183–190
Sousa VS, Teixeira MR (2013) Aggregation kinetics and surface charge of CuO nanoparticles: the influence of pH, ionic strength and humic acids. Environ Chem 10(4):313–322
Wang LF, Wang LL, Ye XD, Li WW, Ren XM, Sheng GP, Wang XK (2013) Coagulation kinetics of humic aggregates in mono-and di-valent electrolyte solutions. Environ Sci Technol 47(10):5042–5049
Wilkinson KJ, Joz‐Roland A, Buffle J (1997) Different roles of pedogenic fulvic acids and aquagenic biopolymers on colloid aggregation and stability in freshwaters. Limnol Oceanogr 42(8):1714–1724
Yang Y, Zhang C, Hu Z (2013) Impact of metallic and metal oxide nanoparticles on wastewater treatment and anaerobic digestion. Environ Sci Process Impacts 15:39–48
Zhang Y, Chen Y, Westerhoff P, Crittenden J (2009) Impact of natural organic matter and divalent cations on the stability of aqueous nanoparticles. Water Res 43:4249–4257
Zhang HY, Smith JA, Oyanedel-Craver V (2012) The effect of natural water conditions on the anti-bacterial performance and stability of silver nanoparticles capped with different polymers. Water Res 46:691–699
Zhao J, Wang Z, Dai Y, Xing B (2013) Mitigation of CuO nanoparticle-induced bacterial membrane damage by dissolved organic matter. Water Res 47:4169–4178
Zhou K, Wang R, Xu B, Li Y (2006) Synthesis, characterization and catalytic properties of CuO nanocrystals with various shapes. Nanotechnology 17:3939
Zhou XH, Huang BC, Zhou T, Liu YC, Shi HC (2015) Aggregation behavior of engineered nanoparticles and their impact on activated sludge in wastewater treatment. Chemosphere 119:568–576
Acknowledgments
We are grateful for the grants from the project supported by the National Science Funds for Creative Research Groups of China (No. 51421006), the National Science Funds for Distinguished Young Scholars (No. 51225901), the National Natural Science Foundation of China (No. 41430751, 51479047, 51479065), the Program for Changjiang Scholars and Innovative Research Team in University (No. IRT13061), the National Science Foundation of China for Excellent Young Scholars (No. 51422902), the Fundamental Research Funds for the Central Universities (No. 2015B22014, No. 2015B05714), and PAPD.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible editor: Thomas D. Bucheli
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 1351 kb)
Rights and permissions
About this article
Cite this article
Miao, L., Wang, C., Hou, J. et al. Effect of alginate on the aggregation kinetics of copper oxide nanoparticles (CuO NPs): bridging interaction and hetero-aggregation induced by Ca2+ . Environ Sci Pollut Res 23, 11611–11619 (2016). https://doi.org/10.1007/s11356-016-6358-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-016-6358-1