Abstract
The occurrence of anti-inflammatory and analgesic pharmaceuticals (AIAPs) in the effluents of 16 hospitals, influent and effluent of wastewater treatment plant (WWTP), the contribution and mass load of each hospital to WWTP influent, and the removal efficiencies in WWTP were investigated. Environmental risk was also evaluated by toxicity tests using organisms from three different trophic levels. Acetaminophen had the highest concentration in summer and winter samples, followed by ketoprofen, ibuprofen, and naproxen. The total daily load of AIAPs detected in influent of WWTP was 1677 mg/day/1000 inhabitants in summer and 5074 mg/day/1000 inhabitants in winter. The contribution of 16 hospitals to the total AIAP load in influent of WWTP was 11.30% in summer and 7.09% in winter. The highest mass loads were calculated as 203 mg/bed.day in general hospital in summer and 300 mg/bed.day in pediatric hospital in winter. The removal efficiencies of AIAPs in WWTP ranged between 13% and 100% in summer and 0.88% and 99% in winter. WWTP is not sufficient to remove all the AIAPs. Diclofenac (in summer), mefenamic acid, indomethacin, and phenylbutazone exhibited poor removal below 50%. The effluents of the WWTP exhibited a low risk for fish and Daphnia magna and an insignificant risk for algae.
Similar content being viewed by others
Introduction
Pharmaceuticals have become an important issue for aquatic ecosystems and human health recently, because these compounds are biologically active substances and resistant to biodegradation in the ecosystem (Daughton and Ternes 1999; Yang et al. 2014). Therefore, pharmaceuticals can be harmful to aquatic ecosystems and human health (Al Aukidy et al. 2012; Tran et al. 2018). Brodin et al. (2013) reported that oxazepam alters behavior and feeding rate of wild European perch at concentrations encountered in effluent-influenced surface waters. The widespread occurrence of antibiotics in the environment with wastewater discharges can result in the development of antimicrobial-resistant bacteria (Fatta-Kassinos et al. 2011).
Anti-inflammatory and analgesic pharmaceuticals (AIAPs) are among the most widely used pharmaceuticals worldwide (AIAPs) and they are commonly administered for the symptomatic treatment of different diseases (Nikolaou et al. 2007). AIAPs were among the therapeutic groups with the highest contribution to the total load of pharmaceuticals originating from hospital effluents (Santos et al. 2013). Sim et al. (2013) investigated 33 pharmaceuticals and a personal care product in river and seawater samples. Antibiotics and non-steroidal anti-inflammatory drugs were identified as dominant compounds because of their relatively higher usage. Diclofenac has the highest acute toxicity within the class of AIAPs and is a potential risk to non-target organisms at concentrations less than 1 μg/L (Aydin et al. 2018; Vieno and Sillanpää 2014). It can cause a decrease in the population of fishes and birds by causing renal failure (Oaks et al. 2004; Hoeger et al. 2005). Due to the increase in diclofenac residues in food sources of Gyps vultures in India, the decline of the population of vultures decrease has been determined by Taggart et al. (2007). Ferrari et al. (2003) determined higher chronic toxicity than acute toxicity for some pharmaceuticals including diclofenac. The investigation on the mixture of anti-inflammatory drugs including diclofenac, ibuprofen, naproxen, and acetylsalicylic acid in water demonstrated a synergistic effect on acute toxicity for Daphnia and algal tests (Cleuvers 2004). Two degradation products of acetaminophen by chlorination in WWTPs were unequivocally identified as the toxic compounds by Bedner and MacCrehan (2005). When ibuprofen has a high acute toxicity and is suspected of endocrine-disrupting activity in human and wildlife (Loraine and Pettigrove 2006), foto-products of naproxen are more toxic than itself in terms of both acute and chronic toxicity (Isidori et al. 2005). Naidoo et al. (2010) detected toxic levels of residual ketoprofen in vulture food supplies.
The most important sources of AIAPs in an aquatic medium are domestic and hospital wastewaters. After consumption of the pharmaceuticals, 30–90% of the active compound is discharged into the sewage system in the form of the parent compound (Lyons 2014). Hospitals can be seen as an important point source of AIAPs to the environment. Gomez et al. (2006) determined about 20 μg/L ibuprofen and 16 μg/L paracetamol in the effluent of small hospitals with 75 beds in Spain. Hospital effluents are generally discharged directly into the public sewage network without any pre-treatment (Verlicchi et al. 2012). Ort et al. (2010) reported that the contribution of hospitals to the pharmaceutical load in urban wastewater represents lower than 15%.
Wastewater treatment plants (WWTPs) do not achieve the complete removal of complex compounds like AIAPs (Gros et al. 2010; Jelic et al. 2011). As seen in Table 1, pKa values of AIAP compounds are ranging between 4.20 and 9.40, except acetylsalicylic acid. For this reason, the removal of these compounds by sorption processes is insufficient; it mainly depends on chemical and biological processes in WWTP. Therefore, the removal of the AIAPs in WWTPs is highly variable (Joss et al. 2005). While ibuprofen or acetaminophen was degraded at quite a high amount with biological or physicochemical processes, diclofenac is the most recalcitrant compound in AIAPs (Joss et al. 2005). Therefore, the WWTP effluents still cause the release of AIAPs into the environment. AIAPs have been detected at levels ranging from ng/L to μg/L in the aquatic medium such as groundwater (Fram and Belitz 2011; Tran et al. 2014a), surface water (Tran et al. 2014a; Kasprzyk-Hordern et al. 2008; Vystavna et al. 2012; Matamoros et al. 2012; Dai et al. 2015; Liu et al. 2015), drinking water (Focazio et al. 2008; Boyd et al. 2003; Carmona et al. 2014; Vulliet et al. 2011), and wastewater (Boyd et al. 2003; Pedrouzo et al. 2011; Carballa et al. 2004; Tran et al. 2014b) and in sludge (Ekpeghere et al. 2017; Ivanová et al. 2018) in many countries. The European Union included several pharmaceuticals (estrone, 17α-ethinyl estradiol, 17β-estradiol, macrolide antibiotics (azithromycin, clarithromycin, erythromycin), methiocarb, neonicotinoids, metaflumizone, amoxicillin, ciprofloxacin) in a watch list of priority substances (European Commission 2018). Today, there are approximately 3000 compounds used as pharmaceuticals and the consumption rate of these drugs has been increasing in every year (Richardson and Ternes 2011).
The Ministry of Health in Turkey has banned the sale of antibiotics without prescription in 2016. However, AIAPs can be bought from a pharmacy without a prescription. There is no legally permissible limit for pharmaceuticals in the waters and very little information has been reported on the amount of AIAPs in wastewater treatment plants in Turkey. This study presents for the first time the occurrence of AIAPs (diclofenac, ibuprofen, naproxen, ketoprofen, mefenamic acid, acetaminophen, acetylsalicylic acid, codeine, indomethacin, phenylbutazone) in the effluents of 16 hospitals (university, pediatric, and general hospitals) in different sizes as well as in the influent and effluent of the urban WWTP receiving domestic wastewater including effluents from the 16 hospitals in Konya, Turkey. The contribution of each hospital effluent to the load of AIAPs into the urban wastewater, the removal efficiency for each AIAPs in the conventional WWTP, and the potential ecotoxicological risks posed by AIAPs to aquatic organisms in the receiving environment were also determined. Despite the increasing number of studies on this field, there is still a need for further regional investigations in order to assess the persistence and potential environmental impact of pharmaceuticals. The manuscript does provide a synthesis of the related data as well as regional context and comparison. Also, Konya WWTP effluent was discharged to the Salt Lake as receiving medium through the main drain channel. The Salt Lake is the second largest lake in the country and is the third saltiest lake in the world. The salt extracted from the lake is used for various purposes such as food industry, animal feed, chemical industry (aluminum, petrochemical, glass, paper, rubber, and textile), defroster, and water treatment. The presence of pharmaceuticals in effluent water may represent a potential hazard to human health.
Material and methods
Chemicals and equipments
All chemicals used for the analyses were of analytical reagent grade. HPLC-grade solvents, HCl (hydrochloric acid, 37%), CH2O2 (formic acid, 98%), and Na2EDTA (ethylenediaminetetraacetic acid disodium salt solution) were purchased from Merck (Darmstadt, Germany). The physico-chemical properties of the target AIAPs are given in Table 1. Acetaminophen, indomethacin, diclofenac, ibuprofen, naproxen, and ketoprofen were acquired from Fluka (Switzerland). Acetylsalicylic acid, phenylbutazone, and mefenamic acid were purchased from Sigma-Aldrich (Missouri, USA) while codeine was obtained from Cerilliant (Texas, USA). Phenylbutazone was prepared with a MeOH/acetonitrile (1/1, v/v), while the other standards were prepared in MeOH. GFF (glass fiber filter), 1.2-μm pore size, was purchased from Whatman (Darmstadt, Germany). Nylon filter with 0.45-μm pore size and PTFE syringe filter with 0.22-μm pore size were obtained from Sartorius (Göttingen, Germany). The Oasis HLB (Hydrophilic Lypophilic, 60 mg, 3 mL) and Oasis MCX (Mixed Polymeric Sorbent, 150 mg, 6 mL) cartridges were acquired from Waters Corporation (Milford, ABD). The high-purity nitrogen gas was obtained from the nitrogen generator (Peak Scientific). HPLC-grade water was obtained from a Milli-Q Plus water purification system (Millipore, Massachusetts, USA).
Wastewater samples
Wastewater samples were collected from the effluents of 16 hospitals and influents and effluents of WWTP. Hospitals and WWTPs are located in Konya in the Central Anatolia region in Turkey. Konya has about 2.2 million populations and an area of 53,850 km2. It is the largest city of Turkey in terms of land and is the seventh most populated city in Turkey. The annual mean temperature is 11.5 °C and the average annual precipitation is about 325 mm. Konya basin is a closed basin. The domestic, hospital and industrial wastewaters collected by combined sewerage system are directed to the central WWTP. WWTP serves approximately 1.2 million population. The effluent wastewater of WWTP is discharged to Konya Main Drainage Channel. Water of the channel is finally discharged to the Salt Lake. Tuz Golu (Salt Lake) is located in the basin and is the second largest lake in the country. There is no specific limit values for treated wastewaters that can be discharged to the Salt Lake. The portion of industrial wastewaters is about 8% of the total amount of wastewaters. The contribution of the hospitals to the total wastewater flow is nearly 3.5%.
Hospitals are different sizes, units and wards. Three of the hospitals are university hospitals and are large hospitals between 903-1298 beds. One hospital is pediatric medium-sized hospital with 376 beds. The remaining hospitals are general hospitals. Four general medium-sized hospitals have beds between 194 and 600, while eight hospitals are small hospital between 27 and 103 beds. Hospitals do not have any wastewater treatment plants. The effluent of each hospital was sampled twice a year (summer, August and winter, January) using portable automatic composite micro-sampler (Durko, Turkey). Every day, 2-h composite samples were collected starting at 8 am, 4 pm, and 8 pm and then samples were combined to provide a final composite sample under dry weather conditions. Samples were transferred to 1-L amber glass bottles and stored at 4 °C until the analysis.
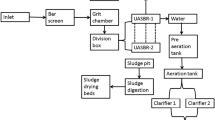
The flow rate of wastewater treated by the WWTP is ca. 300000 m3/day. WWTP consists of a primary treatment including screening, grit removal, and preliminary sedimentation, a secondary treatment including activated sludge and secondary sedimentation, and a disinfection system including ultraviolet radiation. In sampling season, mean pH, electrical conductivity, total suspended solid, and chemical oxygen demand are determined 7.56, 2108 μS/cm, 618 mg/L, and 741 mg/L in influent waters and 7.6, 2077 μS/cm, 306 mg/L, and 322 mg/L in effluent waters in WWTPs, respectively. The influent and effluent wastewater samples (24-h composite) were gathered on the same day with the hospital effluents. The effluent samples were taken according to the constant hydraulic retention time. Samples were collected in amber glass bottles and were stored at 4 °C. The effluent wastewater in WWTP is discharged in the Salt Lake through main drainage channel. The Salt Lake is the second largest lake in the country and is also the third saltiest lake in the world. There are no specific limit values for discharge to the Salt Lake.
Removal efficiency (RE) of individual AIAPs in WWTP was determined by the following Eq. (1). CIWW and CEWW are the concentration of AIAPs measured in WWTP influent and effluent, respectively.
Analytical procedures
At first, wastewater samples for analysis of AIAPs were filtered under a vacuum through GFF followed by a nylon membrane filter. To reduce the binding of AIAP compounds to cation in wastewater, Na2EDTA was added in order to achieve a final Na2EDTA concentration of 0.1% in the wastewaters. Then, aliquots of 200 mL wastewaters were pre-concentrated using solid-phase extraction. Oasis MCX cartridge was used for the enrichment and extraction of acetylsalicylic acid and acetaminophen, Oasis HLB cartridge was used for the other AIAP compounds, while Oasis HLB cartridge was conditioned in sequence with 5 mL of MeOH and 5 mL of ultrapure water; Oasis MCX cartridge was conditioned with 2 × 5 mL of MeOH and 2 × 5 mL of ultrapure water. Then, 200 mL of wastewater sample was loaded into all the cartridges. After sample preconcentration, while Oasis HLB cartridge was rinsed with 5 mL of ultrapure water, Oasis MCX cartridge was rinsed with 2 × 5 mL of ultrapure water. Following drying under vacuum for 10 min, the cartridges were eluted with 4 × 2.5 mL of MeOH. The extracts were evaporated to dryness under nitrogen stream, then re-dissolved using 200 μL of MeOH/water (10/90, v/v). Each sample was analyzed in duplicate.
Analyses of AIAPs were carried out using a 6460 jet stream triple quadrupole mass spectrophotometer (MS) equipped with an electrospray ionization source that was operated in positive and negative modes. To separate the target compounds, an Agilent Poroshell 120 column (100 mm × 3 mm, 2.7 μm) was used. For the positive ionization mode, the mobile phase was ultrapure water containing 0.1% formic acid and 2 mM ammonium formate (A) and MeOH (B). The mobile phase was used ultrapure water containing 10 mM ammonium acetate) (A) and MeOH (B) for the negative ionization mode. For both modes, the flow rate of the mobile phase was 0.5 mL/min, the injection volume was 2 μL, and the column temperature was kept at 35 °C.
The analytical procedure was validated following the method described in previous papers (Aydin et al. 2019). Analytical method validation parameters are given in Table 2. Linearity of the compounds was higher than 0.991. The precision of the compounds as indicated by the RSD values was determined below 4.47 for all AIAP compounds. LOD ranged from 0.003 to 0.227 ng/L while LOQ ranged from 0.011 to 0.757 ng/L. The recoveries of the AIAPs were determined in the range of 72 ± 5 and 96 ± 6% in WWTP influent wastewaters and 75 ± 4 and 95 ± 8% in WWTP effluent wastewaters by analyzing fortified wastewater spiked between 100–5000 ng/L.
Environmental risk assessment
Some AIAP residues can cause adverse effects for aquatic organisms in the environment even at very low concentrations. The anti-inflammatory drug diclofenac can cause a decrease in the population by causing renal failure in fishes and birds (Oaks et al. 2004; Hoeger et al. 2005). Therefore, environmental risk for aquatic organisms should be evaluated by using a hazard quotient (HQ) approach. HQ for individual AIAP residues using three trophic levels representatives of the aquatic ecosystem was calculated using Eq. (2).
MECmax is the measured maximum environmental concentration of each AIAP in hospital effluents and WWTP influent and effluent. In order to obtain the worst-case scenario, MEC was taken the highest concentration ever measured in wastewaters. PNEC is the predicted noneffect environmental concentration (PNEC) of each AIAP given in the literature (EC50 or LC50) and applying an assessment factor (1000) (European Commission 2003). Calculated PNEC values from the reported EC50 values for fish, Daphnia magna, and algae are given in Table 3. If HQ is less than 0.1, no adverse effect is expected (insignificant risk). HQs ranged between 0.1 and 1, and hence, there is a potential adverse effect (low risk). HQs ranged between 1 and 10, and hence, there is a probable adverse effect (moderate risk). If HQ is greater than 10, the adverse effect to aquatic organisms is expected (high risk) (Gomez et al. 2006; Deblonde and Hartemann 2013).
Results and discussion
Occurrence of AIAPs in hospital effluents and influent and effluent wastewaters in WWTP
Table 4 presents the range of concentrations, mean (±standard deviation) and median concentrations of AIAPs in hospital effluents and WWTP influent and effluent. While phenylbutazone in hospitals effluents was not detected in summer, remaining nine AIAP compounds were detected (from 1043 to 203187 ng/L). All AIAPs were detected in the hospital effluents with a concentration range of 2347 and 300285 ng/L in winter. The highest concentrations were determined in the effluents of the general hospital in summer and the pediatric hospital in winter. In the hospital effluents, the analgesic acetaminophen (paracetamol) had the highest concentration in summer and winter, followed by ketoprofen, ibuprofen, and naproxen. Generally, the concentrations of total AIAPs determined in hospital effluents in winter were significantly higher than the concentrations determined in summer, because AIAPs were consumed much more during the winter period. The highest AIAP concentration was detected as 203186 ng/L in the general hospital with 45 beds in summer and 300285 ng/L in the pediatric hospital with 376 beds in winter.
Table 5 shows the concentration ranges reported in hospital effluent for the investigated AIAPs in previous studies. In general, acetaminophen, diclofenac, ibuprofen, naproxen, and ketoprofen are the most often detected AIAP compounds in hospital effluents. The results of determined AIAPs in the studies carried out in different countries show differences among compounds. Generally, acetaminophen was found with the highest concentration. The reason for the high concentration of acetaminophen in wastewater was mainly its large consumption because of the high prevalence of diseases treated with the substance and low Kow value (Table 1), whereas acetaminophen in effluents from four general hospitals in Korea was not detected. When the concentration of acetaminophen in hospital effluents was lower than the concentration (329852 ng/L) found in Norway, ibuprofen (up to 91743 ng/L), naproxen (up to 43360 ng/L), ketoprofen (137671 ng/L), and phenylbutazone (2.38 ng/L) were determined at higher levels. The presence of diclofenac in hospital effluents in Greece (2900 ng/L) and Norway (2737 ng/L) were higher than the values measuremed. The concentrations of codeine (< dl–756 ng/L), acetylsalicylic acid (< dl–236 ng/L), and mefenamic acid (< dl–210 ng/L) were lower than data reported in the literature. Differences in the results observed among studies can be explained with differences in AIAP consumption among countries, prescription, sampling strategies and season, type and size of the hospital, wastewater flow rate, and cultural and geographic factors.
As it can be seen in Table 4, total AIAPs in the WWTP influent were 13421 ng/L in summer and 40594 ng/L in winter while total AIAPs in the WWTP effluent were 785 ng/L in summer and 1264 ng/L in winter. The total AIAP concentration detected in WWTP influent wastewater in winter was higher than the detected concentrations in summer. Acetylsalicylic acid and phenylbutazone were not detected in the WWTP influent and effluent wastewaters in summer. The occurrence of AIAPs in WWTP influent and effluent followed a similar pattern observed in hospital effluents. While naproxen was determined at relatively high concentrations compared to the other compounds in summer, acetaminophen was found with the highest concentration in WWTP influent and effluent wastewaters, followed by naproxen and ibuprofen in winter. AIAPs are frequently used to control moderate or severe acute and chronic pain in human medicine for centuries. Due to frequent repetition of diseases treated with AIAPs, the compounds have been extensively monitored in the different environmental medium.
Biel-Maeso et al. (2018) and Villar-Navarro et al. (2018) reported higher levels of acetaminophen (influent: up to 170000 ng/L, effluent: up to 618 ng/L), ibuprofen (influent: up to 60000 ng/L, effluent: up to 751 ng/L), naproxen (influent: up to 17700 ng/L, effluent: up to 1630 ng/L), and diclofenac (influent: up to 1510 ng/L, effluent: up to 1020 ng/L) in WWTP influent and effluent wastewaters in Spain. Acetaminophen in Greece (52500 ng/L), Canada (500000 ng/L), and USA (62200 ng/L); ibuprofen in Poland (26336 ng/L), Canada (45000 ng/L), and USA (33250 ng/L); and naproxen in Poland (11307 ng/L) and Canada (25000 ng/L) were generally found higher than detected in WWTP influent wastewaters in this study. The concentrations of mefenamic acid, codeine, indomethacin, and phenylbutazone in WWTP influent and effluent wastewaters were also detected lower than those reported in other studies (Table 5). When AIAP concentrations found in the effluent wastewaters of the WWTP were compared with the literature data given in Table 5, in general, higher AIAPs concentration in Spain, Greece, Poland, and Canada were discharged into the receiving environment. The differences of WWTP effluents are related with the wastewater treatment process employed in WWTPs in countries.
Contribution of hospital loads to urban wastewater
In Metcalf and Eddy (2003), the amount of wastewater per person in hospitals is between 660 and 1500 L/day. Usually, a typical value is accepted as 1000 L/day. In order to calculate the mass loadings of AIAPs in hospitals, the wastewater flow of the hospital was accepted as 1000 L/ bed.day. The number of beds and flow rate in hospitals, the AIAP loads, and the contributions to the influent of the WWTP from each hospital are given in Table 6. The total daily load of AIAPs detected in WWTP influent was 1677 mg/day/1000 inhabitants in summer and 5074 mg/day/1000 inhabitants in winter. Analgesic load detected WWTP influent in summer and winter was 387 and 4465 mg/day/1000 inhabitants, while anti-inflammatory load was 1290 and 609 mg/day/1000 inhabitants, respectively. The total mass loads in WWTPs influent for analgesics were determined between 74 and 1149 mg/day/1000 inhabitants in Coimbra, Portugal (Santos et al. 2013), 36081 mg/day/1000 inhabitants (Gallardo-Altamirano et al. 2018), 6-8401 mg/day/1000 inhabitants in four WWTPs influent in Spain (Oliveira et al. 2015). The anti-inflammatory loads in WWTPs influent were detected between 6282 mg/day/1000 inhabitants in Sweden (Zorita et al. 2009), between 80 and 988 mg/day/1000 inhabitants in Coimbra, Portugal (Santos et al. 2013), 3504 mg/day/1000 inhabitants in Spain (Gallardo-Altamirano et al. 2018), and between 0.8 and 6702 mg/day/1000 inhabitants in four WWTP influents in Spain (Oliveira et al. 2015). AIAP loads were ranged from 1300 to 4700 mg/day/1000 inhabitants in seven WWTP influents in Spain (Gros et al. 2007), 11190 mg/day/1000 inhabitants in Italy (Verlicchi et al. 2013), 183 (2.2–2820) mg/day/1000 inhabitants in Greece (Papageorgiou et al. 2016). The total daily AIAP, analgesic, and anti-inflammatory loads obtained in this study were comparatively much lower than those determined in Italy, Spain, and Sweeden, respectively. The differences in mass load in the WWTP influents among countries are related with sampling period, the number of AIAPs analyzed in WWTP influents, and differences between countries such as consumption pattern, hospital type and size, water consumption.
The contribution of each hospital to the total AIAP load the WWTP influent was in the range of 0.01–3.23% in summer and 0–1.74% in winter. While the contribution of 16 hospital loads to WWTP influent was 11.30% in summer and 7.09% in winter, the remaining contribution 88.70% in summer and 92.91% in winter stemmed from the households. The hospital contribution with 900 beds AIAP loads to WWTP influent in Italy was determined 67.4% by Verlicchi et al. (2012). In France, only 13.8% of anticancer drugs are discharged into WWTPs from hospital effluents, while the remaining 86.2% are discharged into the sewerage system diffusely throughout the town and its suburbs (Besse et al. 2012). The contribution of Geneva University Hospitals with 1781 beds to urban AIAP load in Switzerland was determined between 1.2 and 76.6% by Daouk et al. (2016). The contribution of the four hospitals (university hospital with 1456 beds, general hospital with 350 beds, pediatric hospital with 110 beds, maternity hospital with 96 beds) in Portugal was presented 32% for anti-inflammatories and 51% for analgesics (Santos et al. 2013). Oliveira et al. (2015) determined between 1 and 50% for anti-inflammatories and 3 and 80% analgesics for the contribution of the 6 mid-sizes hospitals to the total load in the influent of four WWTPs in the USA. The percentage of paracetamol entering the WWTP services approximately 440,000 people from two Oslo city hospitals was reported as about 12% (Thomas et al. 2007) and 5% while the contribution of other analytes was reported less than 1–2% (Langford and Thomas 2009). Coutu et al. (2013) were reported temporal variability of antibiotics in WWTP serving 200000 residents in Switzerland and contribution from ten hospitals (a total of 1600 beds). The mean annual use ratio of ciprofloxacin in hospitals was 35% and the monthly variable of the ration was determined as low. However, the contribution of hospitals to WWTP influent was temporally and spatially variable. In France, hospital contribution (a medium-sized with 450 beds) to the total load of raw wastewater was presented higher than 15% for paracetamol and 28% for ketoprofen. Despite higher concentrations for some compounds in hospital wastewater, the pharmaceutical contribution of the hospital was determined lower than domestic contribution (Wiest et al. 2018). The contribution of the pollution load derived from hospital (the number of beds 480 ) effluent to WWTP influent serving 420,000 people in Japan was estimated as 0.1% to 15% (Azuma et al. 2019). In general, all hospital contributions of the investigated AIAPs in this study were lower than those presented in the literature.
In summer, the lowest and highest mass loads for AIAPs were calculated as 3.11 mg/bed.day in general hospital with 75 beds and 203 mg/bed.day in general hospital with 45 beds, respectively. In winter, the lower mass load was determined 2.35 mg/bed.day in general hospitals with 27 beds; the higher mass load was detected as 300 mg/bed.day in pediatric hospitals with 376 beds. An average mass load for this therapeutic group in a university hospital with 1000 beds in Spain in June was calculated as 17 mg/bed.day by Mendoza et al. (2015). In Italy, Verlicchi et al. (2012) calculated average mass load 9 mg/bed.day in a medium-sized hospital with 300 beds and 6 mg/bed.day in a large hospital with 900 beds in summer. In Portugal, an average load for AIAPs was determined 43 mg/bed.day in a pediatric hospital with 110 beds, 36 mg/bed.day in a general hospital with 350 beds, 19 mg/bed.day in a university hospital with 1450 beds, and 6 mg/bed.day in a maternity hospital with 96 beds between February and May (2013). In this study, the higher mass load for AIAPs was detected than those presented in Spain, Italy, Portugal. However, there was no relationship between the size of the hospital and the mass load per bed and day of AIAPs in all presented studies. In general, the mass load per bed and day of AIAPs was determined higher in small hospitals.
Potential environmental risks
The HQ values calculated for individual AIAP compounds in hospital and domestic wastewaters are presented in Table 7. HQ values obtained for hospital effluents were higher than the values determined in WWTP wastewaters. In hospital effluents, HQ values of acetaminophen for Daphnia magna in summer and winter was determined higher than 10. Also, HQ value of ibuprofen for fish, Daphnia magna and algae was calculated higher than 10 in winter. The HQs of ibuprofen and naproxen in summer and of naproxen in winter for fish, Daphnia magna, and algae were obtained between 1 and 10. The HQs of some AIAPs including diclofenac for algae, ketoprofen for Daphnia and algae, and acetaminophen and indomethacin for fish, were determined between 0.1 and 1. In WWTP influents, only acetaminophen exhibits a moderate risk for Daphnia magna. Also, ibuprofen and naproxen for all test organisms and acetaminophen for Daphnia magna and algae were exhibited as low risk. In WWTP effluents, no negative effect for all AIAP compounds was determined for aquatic organisms in the receiving medium. However, when the cumulative HQ values in winter were evaluated, there is a low risk for the receiving environment for fish and Daphnia magna. The PNECs used for calculation of HQs were calculated by dividing EC50 values by an assessment factor. If at least one short-term EC50 from each of the three trophic levels are available, an assessment factor should be used as 1000. Although HQ is a deterministic approach to estimating environmental risk at local or regional scales, impacts on the receiving environment should be evaluated on the basis of realistic PNEC values.
Kosma et al. (2014) evaluated the risk characterization level for three trophic levels in the WWTP effluents in Greece and a high chronic risk for diclofenac was determined. HQ values for acetaminophen, diclofenac, ibuprofen, and naproxen in raw water from a medium-sized hospital effluent in Spain were determined higher than 10. The risk assessment results showed that the analgesics and anti-inflammatories pose a high risk to aquatic organisms even by considering dilution and degradation processes. Also, the environmental hazard assessment carried out using PBT (persistent, bioaccumulation, and toxicity) indexes showed that diclofenac and ibuprofen indicate a great potential to danger the environment because they have maximum PBT index value of 9 (Mendoza et al. 2015). Santos et al. (2013) evaluated the HQs obtained for algae, daphnids, and fish in hospitals and WWTP wastewaters in Portugal. According to the results, the ibuprofen and diclofenac can be potentially harmful to fish, especially diclofenac. The potential deleterious effect on aquatic organisms for some analgesic (ibuprofen, diclofenac, and mefenamic acid) was determined in a university hospital in Switzerland (Mendoza et al. 2015). Escher et al. (2011) evaluated the eco-toxicological potential of the 100 pharmaceuticals expected to occur in highest quantities in the wastewater of a general hospital and a psychiatric center in Switzerland. HQmix values for hospital effluents, hospital WWTP effluent, WWTP influent and WWTP effluent were determined 239, 179, 3.2 and 2.4, respectively. Acetaminophen is one of the most commonly consumed analgesics and frequently detected in the aquatic environment. Two of its degradation products by chlorination in WWTPs were unequivocally identified as the toxic compounds by Bedner and MacCrehan (2005). Ibuprofen and naproxen are also widely used analgesics. When ibuprofen has a high acute toxicity (Loraine and Pettigrove 2006), foto-products of naproxen are more toxic than itself both for acute and chronic (Isidori et al. 2005). The excretion rate as unchanged drug of ketoprofen in the urine is 80% (Tauxe-Wuersch et al. 2005). Also, Naidoo et al. (2010) detected toxic levels of residual ketoprofen in vulture food supplies. According to this information given in the literature, some AIAP compounds discharged into the environment can pose risks in the aquatic ecosystem. Furthermore, different pharmaceutical groups, their metabolites and transformation products are available in the aquatic environment and their eco-toxicological effect is unknown.
Removal of AIAPs in WWTP
As seen in Table 8, the removal efficiency of AIAPs varied between 13% (diclofenac) and 100% (codeine) in summer and 0.88 (phenylbutazone) and 98.8% (acetaminophen) in winter. The relatively good removal efficiencies were obtained for acetaminophen, codeine, naproxen, ibuprofen, and ketoprofen compounds. Mefenamic acid, acetylsalicylic acid, indomethacin, and phenylbutazone for the removal efficiencies and were usually lower than 70%. The removal efficiency for ibuprofen and naproxen in winter sampling was determined to be lower than summer sampling. The higher removal efficiencies for ibuprofen and naproxen were achieved in summer than in winter. The water temperature affects biological reaction in the process. The lower water temperature in winter causes lower biodegradation. Therefore, the lower efficiencies have been observed in winter (Xu et al. 2007; Vieno et al. 2007). Solid-liquid partition coefficient (Kd) affects the removal of pharmaceuticals in WWTP. If Log Kd of the compound is lower than 2.7, it weakly adsorbed on the sludge (Ternes et al. 2004). While Log Kd values of mefenamic acid and phenylbutazone are 4.3 and 2.9, respectively, the other AIAPs have the values lower than 2.7. Therefore, these compounds are probably adsorbed and accumulated in the sludge in the WWTP because of its partition coefficient. The main removal process of the other compounds having Log Kd < 2.7 is probably biodegradation in activated sludge in WWTP.
It is seen that in Table 8, the removal efficiency obtained for AIAPs in the study are compatible with the values obtained in the WWTPs with similar treatment processes. The removal efficiencies of acetaminophen in WWTPs including activated sludge were obtained generally high such as 98.4% in Spain, 92% in England, 99% in Canada, 96% in Italy, 97% in Greece, and 100% in Poland, the USA, and Vietnam. The removal rate in the study was 99.3% in summer and 98.8% in winter. While the removal efficiency of naproxen and ibuprofen in Finland, Spain, Canada, Poland, the USA, and Vietnam was over 85%, the removal values in Switzerland, Brazil, England, Italy, and Greece were below 80%. In general, the removal efficiency of diclofenac was obtained as low. While diclofenac was not eliminated in WWTPs in Switzerland and the USA, a negative removal efficiency was obtained in Poland. The existing treatment processes in WWTPs usually are conventional primary and secondary treatments and that these processes are inadequate for the removal of AIAP compounds such as diclofenac, mefenamic acid, acetylsalicylic acid, indomethacin, and phenylbutazone. Radjenovic et al. (2007) compared the removal efficiency of AIAPs by membrane bioreactor and activated sludge processes in WWTP. The higher removal efficiencies for AIAPs were obtained with a membrane bioreactor. However, the efficiency of removal (46.6%) obtained for indomethacin is still insufficient. It is seen that in Table 8, the WWTPs including biologically activated sludge processes are sufficient to remove most of the AIAPs. However, different removal rates for the same compounds in plants containing the same processes can be detected.
Conclusions
Generally, hospital effluent is discharged into the public sewerage system without any treatment. Higher concentrations of AIAPs were determined in hospital effluents than in WWTP influent. The total mass load of AIAPs in WWTP influent was 1677–5074 mg/day/1000 inhabitants. Conventional urban WWTP consists of primary treatment, secondary treatment with activated sludge process, and disinfection process. Removal efficiencies of acetaminophen, ibuprofen, naproxen, and codeine in WWTP varied from more than 97% in summer; the applied conventional wastewater treatment process is not able to efficiently remove of other AIAP compounds, especially in winter. As a result, WWTP effluent wastewaters still cause the release of AIAPs into the environment. Some of the AIAP compounds were determined to cause a negative effect to the environment at concentrations close to determined levels in surface water (Oaks et al. 2004). Even so, AIAPs are still an unregulated emerging compound. Existing WWTPs to remove pharmaceutical compounds in urban wastewaters should be modified by alternative treatment processes such as ozonation, advanced oxidation processes, and activated carbon (Verlicchi et al. 2010).
References
Al Aukidy M, Verlicchi P, Jelic A, Petrovic M, Barcelò D (2012) Monitoring release of pharmaceutical compounds: occurrence and environmental risk assessment of two WWTP effluents and their receiving bodies in the Po Valley, Italy. Sci Total Environ 438:15–25. https://doi.org/10.1016/j.scitotenv.2012.08.061
Aydin S, Aydin ME, Bedük F, Tekinay A, Kilic H (2018) Analysis of diclofenac in water samples using in situ derivatization-vortex-assisted liquid-liquid microextraction with gas chromatography-mass spectrometry. Acta Pharma 68:313–324. https://doi.org/10.2478/acph-2018-0024
Aydin S, Aydin ME, Ulvi A, Kiliç H (2019) Antibiotics in hospital effluents: occurrence, contribution to urban wastewater, removal in a wastewater treatment plant, and environmental risk assessment. Environ Sci Pollut Res 26:544–558. https://doi.org/10.1007/s11356-018-3563-0
Azuma T, Otomo K, Kunitou M, Shimizu M, Hosomaru K, Mikata S, Ishida M, Hisamatsu K, Yunoki A, Mino Y, Hayashi T (2019) Environmental fate of pharmaceutical compounds and antimicrobial resistant bacteria in hospital effluents, and contributions to pollutant loads in the surface waters in Japan. Sci Total Environ 657:476–484. https://doi.org/10.1016/j.scitotenv.2018.11.433
Bedner M, MacCrehan WA (2005) Transformation of acetaminophen by chlorination produces the toxicants 1,4-benzoquinone and N-acetyl-pbenzoquinoneimine. Environ Sci Technol 40:516–522. https://doi.org/10.1021/es0509073
Besse JP, Latour JF, Garric J (2012) Anticancer drugs in surface waters What can say about the occurrence and environmental significance of cytotoxic, cytostatic and endocrine therapy drugs? Environ Int 39:73–86. https://doi.org/10.1016/j.envint.2011.10.002
Biel-Maeso M, Corada-Fernandez C, Lara-Martín PA (2018) Monitoring the occurrence of pharmaceuticals in soils irrigated with reclaimed wastewater. Environ Pollut 235:312–321. https://doi.org/10.1016/j.envpol.2017.12.085
Boyd GR, Reemtsma H, Grimm DA, Mitra S (2003) Pharmaceuticals and personal care products (PPCPs) in surface and treated waters of Louisiana, USA and Ontario, Canada. Sci Total Environ 311:135–149. https://doi.org/10.1016/S0048-9697(03)00138-4
Brodin T, Fick J, Jonsson M, Klaminder J (2013) Dilute concentrations of a psychiatric drug alter behavior of fish from natural populations. Science 339:814–815. https://doi.org/10.1126/science.1226850
Carballa M, Omil M, Lema JM, Llompart M, Garcia-Jares C, Rodriguez I, Gomez M, Ternes T (2004) Behavior of pharmaceuticals, cosmetics and hormones in a sewage treatment plant. Water Res 38:2918–2926. https://doi.org/10.1016/j.watres.2004.03.029
Carmona E, Vicente A, Picó Y (2014) Occurrence of acidic pharmaceuticals and personal care products in Turia River Basin: from waste to drinking water. Sci Total Environ 484:53–63. https://doi.org/10.1016/j.scitotenv.2014.02.085
Cleuvers M (2004) Mixture toxicity of the anti-inflammatory drugs diclofenac, ibuprofen, naproxen, and acetylsalicylic acid. Ecotoxicol Environ Saf 59:309–315. https://doi.org/10.1016/S0147-6513(03)00141-6
Coutu S, Rossi L, Barry DA, Rudaz S, Vemaz N (2013) Temporal variability of antibiotics fluxes in wastewater and contribution from hospitals. PLoS One 8:e53592. https://doi.org/10.1371/journal.pone.0053592
Dai G, Huang J, Chen W, Wang B, Yu G, Deng S (2014) Major pharmaceuticals and personal care products (PPCPs) in wastewater treatment plant and receiving water in Beijing, China, and associated ecological risks. Bull Environ Contam Toxicol 92:655–661. https://doi.org/10.1007/s00128-014-1247-0
Dai G, Wang B, Huang J, Dong R, Deng S, Yu G (2015) Occurrence and source apportionment of pharmaceuticals and personal care products in the Beiyun River of Beijing, China. Chemosphere 119:1033–1039. https://doi.org/10.1016/j.chemosphere.2014.08.056
Daouk S, Chèvre N, Vernaz N, Widmer C, Daali Y, Fleury-Souverain S (2016) Dynamics of active pharmaceutical ingredients loads in a Swiss university hospital wastewaters and prediction of the related environmental risk for the aquatic ecosystems. Sci Total Environ 547:244–253. https://doi.org/10.1016/j.scitotenv.2015.12.117
Daughton CG, Ternes TA (1999) Pharmaceuticals and personal care products in the environment: Agents of subtle change? Environ Health Perspect 107:907–938. https://doi.org/10.1289/ehp.99107s6907
Deblonde T, Hartemann P (2013) Environmental impact of medical prescriptions: assessing the risks and hazards of persistence, bioaccumulation and toxicity of pharmaceuticals. Public Health 127:312–317. https://doi.org/10.1016/j.puhe.2013.01.026
Ekpeghere KI, Lee JW, Kim HY, Shin SK, Oh JE (2017) Determination and characterization of pharmaceuticals in sludge from municipal and livestock wastewater treatment plants. Chemosphere 168:1211–1221. https://doi.org/10.1016/j.chemosphere.2016.10.077
Escher BI, Baumgartner R, Koller M, Treyer K, Lienert J, McArdell CS (2011) Environmental toxicology and risk assessment of pharmaceuticals from hospital wastewater. Water Res 45:75–92. https://doi.org/10.1016/j.watres.2010.08.019
European Commission (2003) Technical Guidance Document on Risk Assessment - Part II - Environmental Risk Assessment. Eur Union 337
European Commission (2018) Commission Implementing Decision (EU) 2018/840 of 5 June 2018 Establishing a Watch List of Substances for Union-wide Monitoring in the Field of Water Policy Pursuant to Directive 2008/105/EC of the European Parliament and of the Council and repealing Commission Implementing Decision (EU) 2015/495
Fatta-Kassinos D, Meric S, Nikolaou A (2011) Pharmaceutical residues in environmental waters and wastewater: current state of knowledge and future research. Anal Bioanal Chem 399:251–275. https://doi.org/10.1007/s00216-010-4300-9
Ferrari B, Paxeus N, Lo Guidice R, Pollio A, Garric J (2003) Ecotoxicological impact of pharmaceuticals found in treated wastewaters: Study of carbamazepine, clofibric acid and diclofenac. Ecotoxicol Environ Saf 55:359–370. https://doi.org/10.1016/S0147-6513(02)00082-9
Ferrari B, Mons R, Vollat BE, Fraysse B, Paxéus N, Lo Giudice R, Pollio A, Garric J (2004) Environmental risk assessment of six human pharmaceuticals: Are the current environmental risk assessment procedures sufficient for the protectıon of the aquatic environment? Environ Toxicol Chem 23:1344–1354. https://doi.org/10.1897/03-246
Focazio MJ, Kolpin DW, Barnes KK, Furlong ET, Meyer MT, Zaugg SD, Barbere LB, Thurman ME (2008) A national reconnaissance for pharmaceuticals and other organic wastewater contaminants in the United States-II, Untreated drinking water sources. Sci Total Environ 402:192–200. https://doi.org/10.1016/j.scitotenv.2008.02.021
Fram MS, Belitz K (2011) Occurrence and concentrations of pharmaceutical compounds in groundwater used for public drinking-water supply in California. Sci Total Environ 409:3409–3417. https://doi.org/10.1016/j.scitotenv.2011.05.053
Gallardo-Altamirano MJ, Maza-Márquez P, Peña-Herrera JM, Rodelas B, Osorio F, Pozo C (2018) Removal of anti-inflammatory/analgesic pharmaceuticals from urban wastewater in a pilot-scale A2O system: Linking performance and microbial population dynamics to operating variables. Sci Total Environ 643:1481–1492. https://doi.org/10.1016/j.scitotenv.2018.06.284
Gomez MJ, Petrovic M, Fernandez-Alba AR, Barcelo D (2006) Determination of pharmaceuticals of various therapeutic classes by solid-phase extraction and liquid chromatography–tandem mass spectrometry analysis in hospital effluent wastewaters. J Chromatogr A 1114:224–233. https://doi.org/10.1016/j.chroma.2006.02.038
Gros M, Petrovic M, Barceló D (2007) Wastewater treatment plants as a pathway for aquatic contamination by pharmaceuticals in the Ebro river basin (northeast Spain). Environ Toxicol Chem 26:1553–1562. https://doi.org/10.1897/06-495R.1
Gros M, Petrovic M, Ginebreda A, Barceló D (2010) Removal of pharmaceuticals during wastewater treatment and environmental risk assessment using hazard indexes. Environ Int 36:15–26. https://doi.org/10.1016/j.envint.2009.09.002
Grung M, Kallqvist T, Sakshaug S, Skurtveit S, Thomas KV (2008) Environmental assessment of Norwegian priority pharmaceuticals based on the EMEA guideline. Ecotoxicol Environ Saf 71:328–340. https://doi.org/10.1016/j.ecoenv.2007.10.015
Guerra P, Kim M, Shah A, Alaee M, Smyth SA (2014) Occurrence and fate of antibiotic, analgesic/anti-inflammatory, and antifungal compounds in five wastewater treatment processes. Sci Total Environ 473-474:235–243. https://doi.org/10.1016/j.scitotenv.2013.12.008
Hoeger B, Köllner B, Dietrich DR, Hitzfeld B (2005) Water-borne diclofenac affects kidney and gill integrity and selected immune parameters in brown trout (Salmo trutta f. fario). Aquat Toxicol 75:53–64. https://doi.org/10.1016/j.aquatox.2005.07.006
Isidori M, Lavorgna M, Nardelli A, Parrella A, Previtera L, Rubino M (2005) Ecotoxicity of naproxen and its phototransformation products. Sci Total Environ 348:93–101. https://doi.org/10.1016/j.scitotenv.2004.12.068
Ivanová L, Mackuľak T, Grabic R, Golovko O, Koba O, Staňová AV, Szabová P, Grenčíková A, Bodík I (2018) Pharmaceuticals and illicit drugs - A new threat to the application of sewage sludge in agriculture. Sci Total Environ 634:606–615. https://doi.org/10.1016/j.scitotenv.2018.04.001
Jelic A, Gros M, Ginebreda A, Cespedes-Sanchez R, Ventura F, Petrovic M, Barcelo D (2011) Occurrence, partition and removal of pharmaceuticals in sewage water and sludge during wastewater treatment. Water Res 45:1165–1176. https://doi.org/10.1016/j.watres.2010.11.010
Jones OAH, Voulvoulis N, Lester JN (2007) The occurrence and removal of selected pharmaceutical compounds in a sewage treatment works utilising activated sludge treatment. Environ Pollut 145:738–744. https://doi.org/10.1016/j.envpol.2005.08.077
Joss A, Keller E, Alder AC, Göbel A, McArdell CS, Ternes T, Siegrist H (2005) Removal of pharmaceuticals and fragrances in biological wastewater treatment. Water Res 39:3139–3152. https://doi.org/10.1016/j.watres.2005.05.031
Kasprzyk-Hordern B, Dinsdal RM, Guwy AJ (2008) The occurrence of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs in surface water in South Wales, UK. Water Res 42:3498–3518. https://doi.org/10.1016/j.watres.2008.04.026
Kasprzyk-Horderna B, Dinsdale RM, Guwy AJ (2009) The removal of pharmaceuticals, personal care products, endocrine disruptors and illicit drugs during wastewater treatment and its impact on the quality of receiving waters. Water Res 43:363–380. https://doi.org/10.1016/j.watres.2008.10.047
Kołecka K, Gajewska M, Stepnowski P, Caban M (2019) Spatial distribution of pharmaceuticals in conventional wastewater treatment plant with Sludge Treatment Reed Beds technology. Sci Total Environ 647:149–157. https://doi.org/10.1016/j.scitotenv.2018.07.439
Kosma CI, Lambropoulou DA, Albanis TA (2010) Occurrence and removal of PPCPs in municipal and hospital wastewaters in Greece. J Hazard Mater 179:804–817. https://doi.org/10.1016/j.jhazmat.2010.03.075
Kosma CI, Lambropoulou DA, Albanisa TA (2014) Investigation of PPCPs in wastewater treatment plants in Greece: Occurrence, removal and environmental risk assessment. Sci Total Environ 466:421–438. https://doi.org/10.1016/j.scitotenv.2013.07.044
Kovalova L, Siegrist H, Singer H, Wittmer A, McArdell CS (2012) Hospital wastewater treatment by membrane bioreactor: Performance and efficiency for organic micropollutant elimination. Environ Sci Technol 46:1536–1545. https://doi.org/10.1021/es203495d
Langford KH, Thomas KV (2009) Determination of pharmaceutical compounds in hospital effluents and their contribution to wastewater treatment works. Environ Int 35:766–770. https://doi.org/10.1016/j.envint.2009.02.007
Lin AYC, Yu TH, Lin CF (2008) Pharmaceutical contamination in residential, industrial, and agricultural waste streams: risk to aqueous environments in Taiwan. Chemosphere 74:131–141. https://doi.org/10.1016/j.chemosphere.2008.08.027
Lindqvist N, Tuhkanen T, Kronberg L (2005) Occurrence of acidic pharmaceuticals in raw and treated sewage and in receiving waters. Water Res 39:2219–2228. https://doi.org/10.1016/j.watres.2005.04.003
Liu J, Lu G, Xie Z, Zhang Z, Li S, Yan Z (2015) Occurrence, bioaccumulation and risk assessment of lipophilic pharmaceutically active compounds in the downstream rivers of sewage treatment plants. Sci Total Environ 511:54–62. https://doi.org/10.1016/j.scitotenv.2014.12.033
Loraine GA, Pettigrove ME (2006) Seasonal variations in concentrations of pharmaceuticals and personal care products in drinking water and reclaimed wastewater in southern California. Environ Sci Technol 40:687–695. https://doi.org/10.1021/es051380x
Lyons G (2014) Pharmaceuticals in the environment: A growing threat to our tap water and wildlife. A CHEM Trust report. http://www.chemtrust.org/wp-content/uploads/CHEM-Trust-Pharma-Dec14.pdf. Accessed 4 Jul 2019
Matamoros V, Arias CA, Nguyen LX, Salvadó V, Brix H (2012) Occurrence and behavior of emerging contaminants in surface water and a restored wetland. Chemosphere 88:1083–1089. https://doi.org/10.1016/j.chemosphere.2012.04.048
Mendoza A, Aceña J, Pérez S, López de Alda M, Barceló D, Gil A, Valcárce Y (2015) Pharmaceuticals and iodinated contrast media in a hospital wastewater: A case study to analyse their presence and characterise their environmental risk and hazard. Environ Res 140:225–241. https://doi.org/10.1016/j.envres.2015.04.003
Metcalf & Eddy (2003) Wastewater Engineering: Treatment and Reuse, 4th edn. McGraw-Hill, New York
Naidoo V, Wolter K, Cromarty D, Diekmann M, Duncan N, Meharg AA, Taggart MA, Venter L, Cuthbert R (2010) Toxicity of non-steroidal anti-inflammatory drugs to Gyps vultures: A new threat from ketoprofen. Biol Lett 6:339–341. https://doi.org/10.1098/rsbl.2009.0818
Nguyen HT, Thai PK, Kaserzon SL, O'Brien JW, Eaglesham G, Mueller JF (2018) Assessment of drugs and personal care products biomarkers in the influent and effluent of two wastewater treatment plants in Ho Chi Minh City, Vietnam. Sci Total Environ 631-632:469–475. https://doi.org/10.1016/j.scitotenv.2018.02.309
Nielsen U, Hastrup C, Klausen MM, Pedersen BM, Kristensen GH, Jansen JLC, Bak SN, Tuerk J (2013) Removal of APIs and bacteria from hospital wastewater by MBR plus O3, O3 + H2O2, PAC or ClO2. Water Sci Technol 67:854–862. https://doi.org/10.2166/wst.2012.645
Nikolaou A, Meric S, Fatta D (2007) Occurrence patterns of pharmaceuticals in water and wastewater environments. Anal Bioanal Chem 387:1225–1234. https://doi.org/10.1007/s00216-006-1035-8
Oaks JL, Gilbert M, Virani MZ, Watson RT, Meteyer CU, Rideout BA, Shivaprasad HL, Ahmed S, Chaudhry MJ, Arshad M, Mahmood S, Ali A, Khan AA (2004) Diclofenac residues as the cause of vulture population decline in Pakistan. Nature 427:630–633. https://doi.org/10.1038/nature02317
Oliveira TS, Murphy M, Mendola N, Wong V, Carlson D, Waring L (2015) Characterization of pharmaceuticals and personal care products in hospital effluent and waste water influent/effluent by direct-injection LC-MS-MS. Sci Total Environ 518-519:459–478. https://doi.org/10.1016/j.scitotenv.2015.02.104
Ort C, Lawrence MG, Reungoat J, Eaglesham G, Carter S, Keller J (2010) Determining the fraction of pharmaceutical residues in wastewater originating from a hospital. Water Res 44:605–615. https://doi.org/10.1016/j.watres.2009.08.002
Paíga P, Correia M, Fernandes MJ, Silva A, Carvalho M, Vieira J, Jorge S, Silva JG, Freire C, Delerue-Matos C (2019) Assessment of 83 pharmaceuticals in WWTP influent and effluent samples by UHPLC-MS/MS: Hourly variation. Sci Total Environ 648:582–600. https://doi.org/10.1016/j.scitotenv.2018.08.129
Papageorgiou M, Kosma C, Lambropoulou D (2016) Seasonal occurrence, removal, mass loading and environmental risk assessment of 55 pharmaceuticals and personal care Escher products in a municipal wastewater treatment plant in Central Greece. Sci Total Environ 543:547–569. https://doi.org/10.1016/j.scitotenv.2015.11.047
Pedrouzo M, Borrull F, Pocurull E, Maria Marcé R (2011) Presence of pharmaceuticals and hormones in waters from sewage treatment plants. Water Air Soil Pollut 217:267–281. https://doi.org/10.1007/s11270-010-0585-8
Perrodin Y, Christine B, Sylvie B, Alain D, Jean-Luc BK, Cécile CO, Audrey R, Elodie B (2013) A priori assessment of ecotoxicological risks linked to building a hospital. Chemosphere 90:1037–1046. https://doi.org/10.1016/j.chemosphere.2012.08.049
Radjenovic J, Petrovic M, Barceló D (2007) Analysis of pharmaceuticals in wastewater and removal using a membrane bioreactor. Anal Bioanal Chem 387:1365–1377. https://doi.org/10.1007/s00216-006-0883-6
Richardson SD, Ternes TA (2011) Water analysis: emerging contaminants and current issues. Anal Chem 83:4614–4648. https://doi.org/10.1021/ac200915r
Rosal R, Rodríguez A, Perdigón-Melón JA, Petre A, García-Calvo E, José Gómez M, Agüera A, Fernández-Alba AR (2010) Occurrence of emerging pollutants in urban wastewater and their removal through biological treatment followed by ozonation. Water Res 44:578–588. https://doi.org/10.1016/j.watres.2009.07.004
Sanderson H, Thomsen M (2009) Comparative analysis of pharmaceuticals versus industrial chemicals acute aquatic toxicity classification according to the United Nations classification system for chemicals. Assessment of the (Q)SAR predictability of pharmaceuticals acute aquatic toxicity and their predominant acute toxic mode-of-action. Toxicol Lett 187:84–93. https://doi.org/10.1016/j.toxlet.2009.02.003
Sanderson H, Johnson DJ, Wilson CJ, Brain RA, Solomon KR (2003) Probabilistic hazard assessment of environmentally occurring pharmaceuticals toxicity to fish, daphnids and algae by ECOSAR screening. Toxicol Lett 144:383–395. https://doi.org/10.1016/S0378-4274(03)00257-1
Santos LH, Gros M, Rodriguez-Mozaz S, Delerue-Matos C, Pena A, Barceló D, Montenegro MC (2013) Contribution of hospital effluents to the load of pharmaceuticals in urban wastewaters: Identification of ecologically relevant pharmaceuticals. Sci Total Environ 461-462:302–316. https://doi.org/10.1016/j.scitotenv.2013.04.077
Sim WJ, Lee JW, Lee ES, Shin SK, Hwang SR, Oh JE (2011) Occurrence and distribution of pharmaceuticals in wastewater from households, livestock farms, hospitals and pharmaceutical manufactures. Chemosphere 82:179–186. https://doi.org/10.1016/j.chemosphere.2010.10.026
Sim WJ, Kim HY, Choi SD, Kwon JH, Oh JE (2013) Evaluation of pharmaceuticals and personal care products with emphasis on anthelmintics in human sanitary waste, sewage, hospital wastewater, livestock wastewater and receiving water. J Hazard Mater 248-249: 219– 227. https://doi.org/10.1016/j.jhazmat.2013.01.007
Stuer-Lauridsen F, Birkved M, Hansen LP, Holten Lützhoft HC, Halling-Sorensen B (2000) Environmental risk assessment of human pharmaceuticals in Denmark after normal therapeutic use. Chemosphere 40:783–793. https://doi.org/10.1016/S0045-6535(99)00453-1
Stumpf M, Ternes TA, Wilken RD, Rodrigues SV, Baumann W (1999) Polar drug residues in sewage and natural waters in the state of Rio de Janeiro, Brazil. Sci Total Environ 225:135–141. https://doi.org/10.1016/S0048-9697(98)00339-8
Suarez S, Lema J, Omil F (2009) Pre-treatment of hospital wastewater by coagulation–flocculation and flotation. Bioresour Technol 100:2138–2146. https://doi.org/10.1016/j.biortech.2008.11.015
Taggart MA, Senacha KR, Green RE, Jhala YV, Raghavan B, Rahmani AR, Cuthbert R, Pain DJ, Meharg AA (2007) Diclofenac residues in carcasses of demostic ungulates available to vultures in India. Environ Int 33:759–765. https://doi.org/10.1016/j.envint.2007.02.010
Tauxe-Wuersch A, De Alencastro LF, Grandjean D, Tarradellas J (2005) Occurrence of several acidic drugs in sewage treatment plants in Switzerland and risk assessment. Water Res 39:1761–1772. https://doi.org/10.1016/j.watres.2005.03.003
Ternes TA, Herrmann N, Bonerz M, Knacker T, Siegrist H, Joss A (2004) A rapid method to measure the solid-water distribution coefficient (Kd) for pharmaceuticals and musk fragrances in sewage sludge. Water Res 38:4075–4084. https://doi.org/10.1016/j.watres.2004.07.015
Thomas KV, Dye C, Schlabach M, Langford KH (2007) Source to sink tracking of selected human pharmaceuticals from two Oslo city hospitals and a wastewater treatment works. J Environ Monit 9:1410–1418. https://doi.org/10.1039/b709745j
Tran NH, Li J, Hu J, Ong SL (2014a) Occurrence and suitability of pharmaceuticals and personal care products as molecular markers for raw wastewater contamination in surface water and groundwater. Environ Sci Pollut Res 21:4727–4740. https://doi.org/10.1007/s11356-013-2428-9
Tran NH, Urase T, Ta TT (2014b) A Preliminary Study on the occurrence of pharmaceutically active compounds in hospital wastewater and surface water in Hanoi, Vietnam. Clean Soil Air Water 42:267–275. https://doi.org/10.1002/clen.201300021
Tran NH, Reinhard M, Yew-Hoong Gin K (2018) Occurrence and fate of emerging contaminants in municipal wastewater treatment plants from different geographical regions. Water Res 133:182–207. https://doi.org/10.1016/j.watres.2017.12.029
Verlicchi P, Galletti A, Petrovic M, Barceló D (2010) Hospital effluents as a source of emerging pollutants: An overview of micropollutants and sustainable treatment options. J Hydrol 389:416–428. https://doi.org/10.1016/j.jhydrol.2010.06.005
Verlicchi P, Al Aukidy M, Galletti A, Petrovic M, Barceló D (2012) Hospital effluent: Investigation of the concentrations and distribution of pharmaceuticals and environmental risk assessment. Sci Total Environ 430:109–118. https://doi.org/10.1016/j.scitotenv.2012.04.055
Verlicchi P, Galletti A, Petrovic M, Barceló D, Al Aukidy M, Zambello E (2013) Removal of selected pharmaceuticals from domestic wastewater in an activated sludge system followed by a horizontal subsurface flow bed-Analysis of their respective contributions. Sci Total Environ 454-455:411–425. https://doi.org/10.1016/j.scitotenv.2013.03.044
Vieno N, Sillanpää M (2014) Fate of diclofenac in municipal wastewater treatment plant-A review. Environ Int 69:28–39. https://doi.org/10.1016/j.envint.2014.03.021
Vieno NM, Härkki H, Tuhkanen T, Kronberg L (2007) Occurrence of pharmaceuticals in river water and theirelimination a pilot-scale drinking water treatment plant. Environ Sci Technol 41:5077–5084. https://doi.org/10.1021/es062720x
Villar-Navarro E, Baena-Nogueras RM, Paniw M, Perales JA, Lara-Martín PA (2018) Removal of pharmaceuticals in urban wastewater: High rate algae pond (HRAP) based technologies as an alternative to activated sludge based processes. Water Res 139:19–29. https://doi.org/10.1016/j.watres.2018.03.072
Vulliet E, Cren-Olivé C, Grenier-Loustalot MF (2011) Occurrence of pharmaceuticals and hormones in drinking water treated from surface waters. Environ Chem Lett 9:103–114. https://doi.org/10.1007/s10311-009-0253-7
Vystavna Y, Huneau F, Grynenko V, Vergeles Y, Celle-Jeanton H, Tapie N (2012) Pharmaceuticals in rivers of two regions with contrasted socio-economic conditions: occurrence, accumulation, and comparison for Ukraine and France. Water Air Soil Pollut 223:2111–2124. https://doi.org/10.1007/s11270-011-1008-1
Wiest L, Chonova T, Bergé A, Baudot R, Bessueille-Barbier F, Ayouni-Derouiche L, Vulliet E (2018) Two-year survey of specific hospital wastewater treatment and its impact on pharmaceutical discharges. Environ Sci Pollut Res 25:9207–9218. https://doi.org/10.1007/s11356-017-9662-5
Xu W, Zhang G, Li X, Zou S, Li P, Hu Z, Li J (2007) Occurrence and elimination of antibiotics at four sewage treatment plants in the Pearl River Delta (PRD), South China. Water Res 41:4526–4534. https://doi.org/10.1016/j.watres.2007.06.023
Yamamoto H, Nakamura Y, Nakamura Y, Kitani C, Imari T, Sekizawa J, Takao Y, Yamashita N, Hirai N, Oda S, Tatarazako N (2007) Initial ecological risk assessment of eight selected human pharmaceuticals in Japan. Environ Sci 14:177–193
Yang G, Fan M, Zhang G (2014) Emerging contaminants in surface waters in China-A short review. Environ Res Lett 9:1–14. https://doi.org/10.1088/1748-9326/9/7/074018
Zorita S, Mårtensson L, Mathiasson L (2009) Occurrence and removal of pharmaceuticals in a municipal sewage treatment system in the south of Sweden. Sci Total Environ 407:2760–2770. https://doi.org/10.1016/j.scitotenv.2008.12.030
Acknowledgments
This work was supported by Turkish Academia of Sciences Awards for Outstanding Young Scientists (TÜBA-GEBIP).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Responsible Editor: Ester Heath
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Aydin, S., Aydin, M.E. & Ulvi, A. Monitoring the release of anti-inflammatory and analgesic pharmaceuticals in the receiving environment. Environ Sci Pollut Res 26, 36887–36902 (2019). https://doi.org/10.1007/s11356-019-06821-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-019-06821-4