Abstract
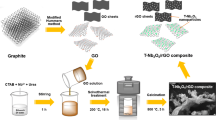
ZnWO4, as an environment-friendly and economic material, has the potential for Li ion batteries (LIB) application. In this paper, a facile method has been developed to synthesize ZnWO4 supported on the reduced graphene oxide (RGO) to improve its LIB performance. The cuboid-like ZnWO4 nanocrystals are prepared by directly adding Na2WO4 powders into the graphene oxide/Zn aqueous solution followed by a hydrothermal treatment. The high-resolution TEM, XRD and XPS characterizations were employed to demonstrate structural information of the as-prepared ZnWO4/RGO hybrids carefully. Besides, we also discussed the LIB properties of the hybrids based on the detailed galvanostatic charge-discharge cycling tests. As a result, the specific capacity of the as-prepared ZnWO4/RGO hybrids reached more than 477.3 mA h g−1 after 40 cycles at a current density of 100 mA g−1 (only less than 159 mA g−1 for bare ZnWO4). During the whole cyclic process, the coulombic efficiency steadily kept the values higher than 90%.
Similar content being viewed by others
References
Li YG, Tan B, Wu YY. Mesoporous Co3O4 nanowire arrays for lithium ion batteries with high capacity and rate capability. Nano Lett, 2008, 8: 265–270
Lou XW, Wang Y, Yuan C, Lee JY, Archer LA. Template-free synthesis of SnO2 Hollow nanostructures with high lithium storage capacity. Adv Mater, 2006, 18: 2325–2329
Wang ZY, Zhou L, Lou XW. Metal oxide hollow nanostructures for lithium-ion batteries. Adv Mater, 2012, 24: 1903–1911
Wang X, Guan H, Chen SM, Li HQ, Zhai TY, Tang DM, Bando Y, Golberg D. Self-stacked Co3O4 nanosheets for high-performance lithium ion batteries. Chem Commun, 2011, 47 (45): 12280–12282
Li XY, Huang XL, Liu DP, Wang X, Song SY, Zhou L, Zhang HJ. Synthesis of 3D hierarchical Fe3O4/graphene composites with high lithium storage capacity and for controlled drug delivery. J Phys Chem C, 2011, 115: 21567
Fu YS, Wang X. Magnetically separable ZnFe2O4-graphene catalyst and its high photocatalytic performance under visible light irradiation. Ind Eng Chem Res, 2011, 50: 7210–7218
Feng JK, Lai MO, Lu L. Zn2GeO4 nanorods synthesized by low-temperature hydrothermal growth for high-capacity anode of lithium battery. Electro Comm, 2011, 13: 287–289
Chen YJ, Qu BH, Lin Mei, Lei DN, Chen LB, Li QH, Wang TH. Synthesis of ZnSnO3 mesocrystals from regular cube-like to sheet-like structures and their comparative electrochemical properties in Li-ion batteries. J Mater Chem, 2012, 22: 25373–25379
Shim HW, Cho IS, Hong KS, Lim AH, Kim DW. Wolframite-type ZnWO4 nanorods as new anodes for Li-ion batteries. J Phys Chem C, 2011, 115: 16228–16233
Wang HL, Cui LF, Yang Y, Casalongue HS, Robinson JT, Liang YY, Cui Y, Dai HJ. Mn3O4-Graphene hybrid as a high-capacity anode material for lithium ion batteries. J Am Chem Soc, 2010, 132: 13978–13980
Li L, Guo ZP, Du AJ, Liu HK. Rapid microwave-assisted synthesis of Mn3O4-graphene nanocomposite and its lithium storage properties. J Mater Chem, 2012, 22: 3600–3605
Lee JE, Yu SH, Lee DJ, Lee DC, Han SI, Sung YE, Hyeon T. Facile and economical synthesis of hierarchical carbon-coated magnetite nanocomposite particles and their applications in lithium ion battery anodes. Energy Environ Sci, 2012, 5: 9528–9533
Muraliganth T, Murugan AV, Manthiram A. Facile synthesis of carbon-decorated single-crystalline Fe3O4 nanowires and their application as high performance anode in lithium ion batteries. Chem Comm, 2009: 7360–7362
Ding SJ, Luan DY, Boey FYC, Chen JS, Lou XW. SnO2 nanosheets grown on graphene sheets with enhanced lithium storage properties. Chem Comm, 2011, 47: 7155
Wang ZY, Zhou L, Lou XW. Metal oxide hollow nanostructures for lithium-ion batteries. Adv Mater, 2012, 24: 1903–1911
Bi JH, Wu L, Li ZH, Ding ZX, Wang XX, Fu XZ. A facile microwave solvothermal process to synthesize ZnWO4 nanoparticles. J Alloys Compd, 2009, 480: 684–688
Zhao W, Song XY, Chen GZ, Sun SX. One-step template-free synthesis of ZnWO4 hollow clusters. J Mater Sci, 2009, 44: 3082–3087
Lin J, Zhu YF. Controlled synthesis of the ZnWO4 nanostructure and effects on the photocatalytic performance. Inorg Chem, 2007, 46: 8372–8378
Seng KH, Guo ZP, Chen ZX, Liu HK. SnSb/graphene composite as anode materials for lithium ion batteries. Adv Sci Lett, 2011, 4: 18–23
Wagner CD, Riggs WM, Davis LE, Moulder JF, Muilemberg GE. Hand Book of X-Ray Photoelectron Spectroscopy. Minnesota: Perkin-Elmer Corporation, 1979
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Wang, X., Li, B., Liu, D. et al. ZnWO4 nanocrystals/reduced graphene oxide hybrids: Synthesis and their application for Li ion batteries. Sci. China Chem. 57, 122–126 (2014). https://doi.org/10.1007/s11426-013-4983-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-013-4983-9