Abstract
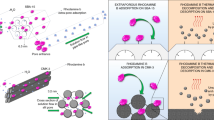
An extremely effortless method was applied for successful synthesis of mesoporous carbonaceous materials (MCMs) using well-ordered mesoporous silica as template. Various characterizations (scanning electron microscopy (SEM), transmission electron microscopy (TEM), X-ray diffraction (XRD), Fourier transform infrared spectroscopy (FT-IR), Raman, X-ray photoelectron spectroscopy (XPS), Brunner-Emmet-Teller (BET) and Zeta potential) confirmed that MCMs had large surface area, uniform pore size distribution, and abundant oxygen-containing functional groups. The batch techniques were employed to study U(VI) adsorption on MCMs under a wide range of experiment conditions. The adsorption kinetics of U(VI) onto MCMs were well-fitted by pseudo-second-order kinetic model, indicating a chemisorption process. The excellent adsorption capacity of MCMs calculated from the Langmuir model was 293.95 mg g−1 at pH 4.0. The FT-IR and XPS analyses further evidenced that the binding of U(VI) onto MCMs was ascribed to the plentiful adsorption sites (–OH and –COOH groups) in the internal mesoporous structure, which could efficiently trap guest U(VI) ions. The results presented herein revealed that MCMs were ideal adsorbents in the efficient elimination of uranium or other lanthanides/actinides from aqueous solutions, which would play an important role in environmental pollution management application.
Similar content being viewed by others
References
Liu W, Dai X, Bai Z, Wang Y, Yang Z, Zhang L, Xu L, Chen L, Li Y, Gui D, Diwu J, Wang J, Zhou R, Chai Z, Wang S. Environ Sci Technol, 2017, 51: 3911–3921
Xu L, Zheng T, Yang S, Zhang L, Wang J, Liu W, Chen L, Diwu J, Chai Z, Wang S. Environ Sci Technol, 2016, 50: 3852–3859
Sun Y, Ding C, Cheng W, Wang X. J Hazard Mater, 2014, 280: 399–408
Wang Y, Liu Z, Li Y, Bai Z, Liu W, Wang Y, Xu X, Xiao C, Sheng D, Diwu J, Su J, Chai Z, Albrecht-Schmitt TE, Wang S. J Am Chem Soc, 2015, 137: 6144–6147
Sheng D, Zhu L, Xu C, Xiao C, Wang Y, Wang Y, Chen L, Diwu J, Chen J, Chai Z, Albrecht-Schmitt TE, Wang S. Environ Sci Technol, 2017, 51: 3471–3479
Xie J, Wang Y, Liu W, Yin X, Chen L, Zou Y, Diwu J, Chai Z, Albrecht-Schmitt TE, Liu G, Wang S. Angew Chem Int Ed, 2017, 56: 7500–7504
Suresh A, Srinivasan TG, Rao PRV. Solvent Extr Ion Exch, 1994, 12: 727–744
Kraus KA, Moore GE, Nelson F. J Am Chem Soc, 1956, 78: 2692–2695
Tang Y, Reeder RJ. Geochim Cosmochim Acta, 2009, 73: 2727–2743
Zhu L, Xiao C, Dai X, Li J, Gui D, Sheng D, Chen L, Zhou R, Chai Z, Albrecht-Schmitt TE, Wang S. Environ Sci Technol Lett, 2017, 4: 316–322
Brooks SC, Fredrickson JK, Carroll SL, Kennedy DW, Zachara JM, Plymale AE, Kelly SD, Kemner KM, Fendorf S. Environ Sci Technol, 2003, 37: 1850–1858
Zheng T, Yang Z, Gui D, Liu Z, Wang X, Dai X, Liu S, Zhang L, Gao Y, Chen L, Sheng D, Wang Y, Diwu J, Wang J, Zhou R, Chai Z, Albrecht-Schmitt TE, Wang S. Nat Commun, 2017, 8: 15369
Sun Y, Yang S, Chen Y, Ding C, Cheng W, Wang X. Environ Sci Technol, 2015, 49: 4255–4262
Wang X, Fan Q, Yu S, Chen Z, Ai Y, Sun Y, Hobiny A, Alsaedi A, Wang X. Chem Eng J, 2016, 287: 448–455
Zhang R, Chen C, Li J, Wang X. J Colloid Interface Sci, 2015, 460: 237–246
Sylwester ER, Hudson EA, Allen PG. Geochim Cosmochim Acta, 2000, 64: 2431–2438
Zeng H, Singh A, Basak S, Ulrich KU, Sahu M, Biswas P, Catalano JG, Giammar DE. Environ Sci Technol, 2009, 43: 1373–1378
Mellah A, Chegrouche S, Barkat M. J Colloid Interface Sci, 2006, 296: 434–441
Kumar S, Loganathan VA, Gupta RB, Barnett MO. J Environ Manage, 2011, 92: 2504–2512
Shao D, Jiang Z, Wang X, Li J, Meng Y. J Phys Chem B, 2009, 113: 860–864
Sun Y, Wu ZY, Wang X, Ding C, Cheng W, Yu SH, Wang X. Environ Sci Technol, 2016, 50: 4459–4467
Darmstadt H, Roy C, Kaliaguine S, Kim TW, Ryoo R. Chem Mater, 2003, 15: 3300–3307
Tian G, Geng J, Jin Y, Wang C, Li S, Chen Z, Wang H, Zhao Y, Li S. J Hazard Mater, 2011, 190: 442–450
Jiang H, Ma J, Li C. Adv Mater, 2012, 24: 4197–4202
Lee JE, Lee N, Kim H, Kim J, Choi SH, Kim JH, Kim T, Song IC, Park SP, Moon WK, Hyeon T. J Am Chem Soc, 2010, 132: 552–557
Liu R, Zhang Y, Zhao X, Agarwal A, Mueller LJ, Feng P. J Am Chem Soc, 2010, 132: 1500–1501
Li C. Catal Rev, 2004, 46: 419–492
Kruk M, Jaroniec M, Kim TW, Ryoo R. Chem Mater, 2003, 15: 2815–2823
Han YJ, Kim JM, Stucky GD. Chem Mater, 2000, 12: 2068–2069
Schumacher K, Grü n M, Unger KK. Microporous Mesoporous Mater, 1999, 27: 201–206
Wu Z, Webley PA, Zhao D. Langmuir, 2010, 26: 10277–10286
Zhuang X, Wan Y, Feng C, Shen Y, Zhao D. Chem Mater, 2009, 21: 706–716
Hartmann M, Vinu A, Chandrasekar G. Chem Mater, 2005, 17: 1169–1176
Vinu A, Miyahara M, Sivamurugan V, Mori T, Ariga K. J Mater Chem, 2005, 15: 5122–5127
Silva R, Voiry D, Chhowalla M, Asefa T. J Am Chem Soc, 2013, 135: 7823–7826
Ren X, Li J, Tan X, Shi W, Chen C, Shao D, Wen T, Wang L, Zhao G, Sheng G, Wang X. Environ Sci Technol, 2014, 48: 5493–5500
Stankovich S, Piner RD, Nguyen SBT, Ruoff RS. Carbon, 2006, 44: 3342–3347
Sun Y, Wang Q, Chen C, Tan X, Wang X. Environ Sci Technol, 2012, 46: 6020–6027
Chen H, Wang X, Li J, Wang X. J Mater Chem A, 2015, 3: 6073–6081
Wu Z, Li W, Webley PA, Zhao D. Adv Mater, 2012, 24: 485–491
Wang Y, Gu Z, Yang J, Liao J, Yang Y, Liu N, Tang J. Appl Surf Sci, 2014, 320: 10–20
Sun Y, Shao D, Chen C, Yang S, Wang X. Environ Sci Technol, 2013, 47: 9904–9910
Wang YQ, Zhang ZB, Liu YH, Cao XH, Liu YT, Li Q. Chem Eng J, 2012, 198-199: 246–253
Grenthe I, Fugar J, Konings RJM, Lemire R, Muller AB, Nguyen TC, Wanner H. Chemical Thermodynamics of Uranium. Amsterdam: North-Holland, 1992
Wen T, Wang X, Wang J, Chen Z, Li J, Hu J, Hayat T, Alsaedi A, Grambow B, Wang X. Inorg Chem Front, 2016, 3: 1227–1235
Dong W, Brooks SC. Environ Sci Technol, 2006, 40: 4689–4695
Zhao G, Wen T, Yang X, Yang S, Liao J, Hu J, Shao D, Wang X. Dalton Trans, 2012, 41: 6182–6188
Sprynskyy M, Kovalchuk I, Buszewski B. J Hazard Mater, 2010, 181: 700–707
Lim SF, Zheng YM, Zou SW, Chen JP. Environ Sci Technol, 2008, 42: 2551–2556
Manos MJ, Kanatzidis MG. J Am Chem Soc, 2012, 134: 16441–16446
Acknowledgments
This work was supported by the National Natural Science Foundation of China (91326202, 21577032), the Fundamental Research Funds for the Central Universities (JB2015001, JB2017057), the Jiangsu Provincial Key Laboratory of Radiation Medicine and Protection and the Priority Academic Program Development of Jiangsu Higher Education Institutions. X. Wang acknowledges the CAS Interdisciplinary Innovation Team of Chinese Academy of Sciences.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, C., Li, X., Chen, Z. et al. Synthesis of ordered mesoporous carbonaceous materials and their highly efficient capture of uranium from solutions. Sci. China Chem. 61, 281–293 (2018). https://doi.org/10.1007/s11426-017-9132-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11426-017-9132-7