Abstract
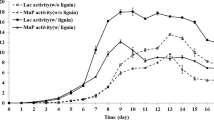
An ultrafiltered low-molecular-weight preparation of chelating compounds was isolated from a wood-containing culture of the white-rot basidiomycete Coriolus versicolor. This preparation could chelate Fe3+ and reduce Fe3+ to Fe2+, demonstrating that the substance may serve as a ferric chelator, oxygen-reducing agent, and redox-cycling molecule, which would include functioning as the electron transport carrier in Fenton reaction. Lignin was treated with the iron-binding chelator and the changes in structure were investigated by 1H-NMR, 13C-NMR, difference spectrum caused by ionization under alkaline conditions and nitrobenzene oxidation. The results indicated that the iron-binding chelator could destroy the β-O-4 bonds in etherified lignin units and insert phenolic hydroxyl groups. The low-molecular-weight chelator secreted by C. versicolor resulted in new phenolic substructures in the lignin polymer, making it susceptible to attack by laccase or manganese peroxidase. Thus, the synergic action of the iron-binding chelator and the lignocellulolytic enzymes made the substrate more accessible to degradation.
Similar content being viewed by others
References
Kirk T K, Farrell R L. Enzymatic “combustion”: The microbial degradation of lignin. Annu Rev Microbiol, 1987, 41: 465–505
Shingo k, Masanori A, Noriko O, et al. Degradation of a non-phenolic β-O-4 substructure and of polymeric lignin model compounds by laccase of Coriolus versicolor in the presence of 1-hydroxybenzotriazole. FEMS Lett, 1999, 170(1): 51–57
Enoki A, Tanaka H, Fuse G. Relationship between degradation of wood and production of H2O2-producing or one-electron oxidases by brown-rot fungi. Wood Sci Technol, 1989, 23: 1–12
Rimko T H, Pauline J M. Oxidative mechanisms involved in lignin degradation by white-rot fungi. Chem Rev 2001, 101: 3397–3413
Hammel K E, Kapich A N, Jensen K A, et al. Reactive oxygen species as agents of wood decay by fungi. Enzyme Microb Technol, 2002, 30(4): 445–453
Srebotnik E, Messner K, Foisner R. Penetrability of white rot-degraded pine wood by the lignin peroxidase of Phanerochaete chrysosporium. Appl Environ Microbiol, 1988, 54(11): 2608–2614
Goodell B, Jellison J, Liu J, et al. Low molecular weight chelators and phenolic compounds isolated from wood decay fungi and their role in the fungal biodegradation of wood. J Biotechnol, 1997, 53: 133–162
Tanaka H, Itakura S, Enoki A. Hydroxyl radical generation by an extracellular low-molecular-weight substance and phenol oxidase activity during wood degradation by the white-rot basidiomycete Trametes versicolor. J Biotechnol, 1999, 75(1): 57–70
Tanaka H, Itakura S, Enoki A. Phenol oxidase activity and one-electron oxidation activity in wood degradation by soft-rot deuteromycetes. Holzforschung, 2000, 54(5): 463–468
Hirano T, Tanaka H, Enoki A. Relationship between hydroxyl radicals and degradation of wood by the brown-rot fungus, Tyromyces palustris. Holzforschung, 1996, 50: 541–548
Liu J, Shen X Y, Gao P J. Short fibre formation during cellulose degradation by celluloytic fungi. Biotechnol Lett, 1996, 18(11): 1235–1240
Yang W H, Liu J, Gao P J, et al. Function of a low molecular peptide generated by cellulolytic fungi for the degradation of native cellulose. Biotechnol Lett, 2004, 26: 1799–1802
Wang W, Gao P J. A peptide-mediated and hydroxyl radical HO·-involved oxidative degradation of cellulose by brown-rot fungi. Biodegradation, 2002, 13: 383–394
Wang W, Huang F, Gao P J. Lignin degradation by a novel peptide, Gt factor, from brown rot fungus Gloeophyllum trabeum. Biotechnol J, 2006, 1: 447–453
Hu M, Zhang W C, Gao P J, et al. Purification and characteristics of a low-molecular-weight peptide possessing oxidative capacity for phenol from Phanerochaete chrysosporium. Sci China Ser C-Life Sci, 2006, 49(3): 243–250
Huang F, Gao P J. Using cyclic liquid-liquid extraction method for isolation and identification of relative compounds during lignin biodegradation. Sci China Ser E-Tech Sci, 1999, 42: 644–651
Schwyn B, Neilands J B. Universal chemical assay for the detection and determination siderophores. Anal Biochem, 1987, 160: 47–56
Gibbs C R. Characterization and application of ferrozine iron reagent as a ferrous iron indicator. Anal Chem, 1976, 48: 1197–1201
Chen C L. Methods in Lignin Chemistry. Heidelberg: Springer Series in Wood Sciences, 1992. 215–319
Arantes V, Milagres A M. The effect of a catecholate chelator as a redox agent in Fenton-based reactions on degradation of lignin-model substrates and on COD removal from effluent of an ECF kraft pulp mill. J Hazard Mater, 2007, 141(1): 273–279
Huang F, Fang J, Gao P J, et al. Synergistic effects of cellubiose dehydrogenase and manganese-dependent peroxidases during lignin degradation. Chin Sci Bull, 2001, 46(23): 1956–1962
Kenneth A, Carl J, Kenneth E. Pathways for extracellular fenton chemistry in the brown rot basidiomycete Gloeophyllum trabeum. Appl Environ Microbiol 2001, 67: 2705–2711
Fang J, Huang F, Gao P J. Optimization of cellobiose dehydrogenase production by Schizophyllum commune and effect of the enzyme on kraft pulp bleaching by ligninases. Process Biochem, 1999, 34(9): 957–961
Eggert C, Temp U, Dean J F D, et al. Laccase-mediated formation of the phenoxazinone derivative, cinnabarinic acid. FEMS Lett, 1995, 376(3): 202–206
Author information
Authors and Affiliations
Corresponding authors
Additional information
Supported by the National Natural Science Foundation of China (Grant Nos. 30170027 and 30371136)
Rights and permissions
About this article
Cite this article
Wang, L., Yan, W., Chen, J. et al. Function of the iron-binding chelator produced by Coriolus versicolor in lignin biodegradation. Sci. China Ser. C-Life Sci. 51, 214–221 (2008). https://doi.org/10.1007/s11427-008-0033-9
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11427-008-0033-9