Abstract
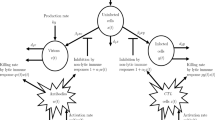
There are numerous examples of human pathogens which persist in environmental reservoirs while infectious outbreaks remain rare. In this manuscript, we consider the dynamics of infectious diseases for which the primary mode of transmission is indirect and mediated by contact with a contaminated reservoir. We evaluate the realistic scenario in which the number of ingested pathogens must be above a critical threshold to cause infection in susceptible individuals. This minimal infectious dose is a consequence of the clearance effect of the innate immune system. Infected individuals shed pathogens back into the aquatic reservoir, indirectly increasing the transmittability of the pathogen to the susceptible. Building upon prior works in the study of cholera dynamics, we introduce and analyze a family of reservoir mediated SIR models with a threshold pathogen density for infection. Analyzing this family of models, we show that an outbreak can result from noninfinitesimal introductions of either infected individuals or additional pathogens in the reservoir. We devise two new measures of how likely it is that an environmentally persistent pathogen will cause an outbreak: (i) the minimum fraction of infected individuals; and (ii) the minimum fluctuation size of in-reservoir pathogens. We find an additional control parameter involving the shedding rate of infected individuals, which we term the pathogen enhancement ratio, which determines whether outbreaks lead to epidemics or endemic disease states. Thus, the ultimate outcome of disease is controlled by the strength of fluctuations and the global stability of a nonlinear dynamical system, as opposed to conventional analysis in which disease reflects the linear destabilization of a disease free equilibrium. Our model predicts that in the case of waterborne diseases, suppressing the pathogen density in aquatic reservoirs may be more effective than minimizing the number of infected individuals.
Similar content being viewed by others
References
Bove, F.J., Fulcomer, M.C., Klotz, J.B., Esmart, J., Dufficy, E.M., Savrin, J.E., 1995. Public drinking water contamination and birth outcomes. Am. J. Epidemiol. 141, 850–62.
Brayton, P.R., Tamplin, M.L., Huq, A., Colwell, R.R., 1987. Enumeration of vibrio cholerae O1 in Bangladesh waters by fluorescent-antibody direct viable count. Appl. Environ. Microbiol. 53, 2862–865.
Capasso, V., Paveri-Fontana, S.L., 1979. A mathematical model for the 1973 cholera epidemic in the European Mediterranean region. Rev. Epidém. Santé Pub. 27, 121–32.
Chtsulo, L., Engles, D., Montresor, A., Savioli, L., 2000. The global status of schistosomiasis and its control. Acta Trop. 77, 41–1.
Codeço, C.T., 2001. Endemic and epidemic dynamics of cholera: the role of the aquatic reservoir. BMC Infect. Dis. 1, 1.
Colwell, R.R., Bryaton, P., Herrington, D., Tall, B., Huq, A., Levine, M.M., 1996. Viable but nonculturable vibrio cholerae revert to a cultivable state in the human intestine. World J. Microbiol. Biotechnol. 12, 28–1.
Craig, M.H., Snow, R.W., le Sueur, D., 1999. A climate-based distribution model of malaria transmission in sub-Saharan Africa. Parasitol. Today 15, 105–11.
Dietz, K., 1993. The estimation of the basic reproduction number for infectious diseases. Stat. Meth. Med. Res. 2, 23–1.
DuPont, H.L., Chappell, C.L., Sterling, C.R., Okhuysen, P.C., Rose, J.B., Jakubowski, W., 1995. The infectivity of cryptosporidium parvum in healthy volunteers. N. Eng. J. Med. 332, 855–59.
Estes, M.K., Palmer, E.L., Obijeski, J.F., 1983. Rotavirus: a review. Curr. Top. Microbiol. Immunol. 105, 123–84.
Fields, B.S., Benson, R.F., Besser, R.E., 2002. Legionella and legionnaires’ disease: 25 years of investigation. Clin. Microbiol. Rev. 15, 506–26.
Häder, D.P., Kumar, H.D., Smith, R.C., Worrest, R.C., 1998. Effects on aquatic ecosystems. J. Photochem. Photobiol. B: Biol. 46, 53–8.
Hartley, D.M., Morris, J.G., Smith, D.L., 2006. Hyperinfectivity: A critical element in the ability of v. cholerae to cause epidemics? PLoS Med. 3, e7.
Holling, C.S., 1959. The components of predation as revealed by a study of small-mammal predation of the European pine sawfly. Can. Entomol. 91, 293–20.
Jensen, P.K., Ensink, J.H.J., Jayasinghe, G., van de Hoek, W., Cairncross, S., Dalsgaard, A., 2002. Domestic transmission routes of pathogens: the problem of in-house contamination of drinking water during storage in developing countries. Trop. Med. Int. Health 7, 604–09.
Jensen, M.A., Faruque, S.M., Mekalanos, J.J., Levin, B.R., 2006. Modeling the role of bacteriophage in the control of cholera outbreaks. Proc. Natl. Acad. Sci. USA 103, 4652–657.
Kaper, J.B., Morris Jr., J.G., Levine, M.M., 1995. Cholera. Clin. Microbiol. Rev. 8, 48–6.
Kermack, W.O., McKendrik, A.G., 1927. A contribution to the mathematical theory of epidemics. Proc. R. Soc. Lond. A 115, 700–21.
LeChevallier, M.W., Norton, W.D., Lee, R.G., 1991. Giardia and cryptosporidium spp. in filtered drinking water supplies. Appl. Environ. Microbiol. 57, 2617–621.
Levine, M.M., Black, R.E., Clements, M.L., Nalin, D.R., Cisneros, L., Finkelstein, R.A., 1981. Volunteer studies in development of vaccines against cholera and enterotoxigenic escherichia coli: a review. In: Holme, T., Holmgren, J., Merson, M.H., Mollby, R. (Eds.), Acute Enteric Infections in Children. New Prospects for Treatment and Prevention, pp. 443–59. Elsevier/North-Holland Biomedical Press, Amsterdam.
Macdonald, G., 1952. The analysis of equilibrium in malaria epidemiology. Trop. Dis. Bull. 49, 813–29.
Mourino-Pérez, R.R., Worden, A.Z., Azam, F., 2003. Growth of vibrio cholerae O1 in red tide waters off California. Appl. Environ. Microbiol. 69, 6923–931.
Murphy, K.M., Travers, P., Walport, M., 2007. Janeway’s Immunobiology, 7th edn. Garland Science.
Pascual, M., Rodó, X., Ellner, S.P., Colwell, R., Bouma, M.J., 2000. Cholera dynamics and El Niño-Southern oscillation. Science 289, 1766–769.
Real, L., 1977. The kinetics of functional response. Am. Nat. 111, 289–00.
Rendtorff, R.C., 1954. The experimental transmission of human intestinal protozoan parasites: Giardia lamblia cysts given in capsules. Am. J. Hyg. 59, 209–20.
Rose, J.B., 1997. Environmental ecology of crptosporidiumm and public health implications. Annu. Rev. Public Health 18, 135–61.
Ross, R., 1908. Report on the Prevention of Marlaria in Mauritius. Waterlow and Sons Ltd.
Strogatz, S.H., 1994. Nonlinear Dynamics and Chaos: With Applications to Physics, Biology, Chemistry, and Engineering. Perseus Books.
Ward, R.L., Bernstein, D.I., Young, E.C., Sherwood, J.R., Knowlton, D.R., Shiff, G.M., 1986. Human rotavirus studies in volunteers: determination of infectious dose and serological response to infection. J. Infectious Dis. 154, 871–80.
Webster, A.D., 1980. Giardiasis and immunodeficiency diseases. Trans. R. Soc. Trop. Med. Hyg. 74, 440–43.
White, P.O., Fenner, F.J., 1994. Medical Virology, 4th edn. Academic, San Diego.
Wolfe, M.S., 1992. Giardiasis. Clin. Microbiol. Rev. 5, 93–00.
Wolfe, N.D., Dunovan, C.P., Diamond, J., 2007. Origins of major human infectious diseases. Nature 447, 279–83.
Yoganathan, D., Rom, W.N., 2001. Medical aspects of global warming. Am. J. Ind. Med. 40, 199–10.
Zaki, S.R., et al., 1995. Hantavirus pulmonary syndrome—pathogenesis of an emerging infectious disease. Am. J. Pathol. 146, 552–79.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Joh, R.I., Wang, H., Weiss, H. et al. Dynamics of Indirectly Transmitted Infectious Diseases with Immunological Threshold. Bull. Math. Biol. 71, 845–862 (2009). https://doi.org/10.1007/s11538-008-9384-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-008-9384-4