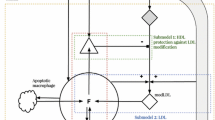
Abstract
Atherosclerosis, the leading cause of death in the US, is a disease in which a plaque builds up inside the arteries. The low density lipoprotein (LDL) and high density lipoprotein (HDL) concentrations in the blood are commonly used to predict the risk factor for plaque growth. In a recent paper (Hao and Friedman in Plos One e90497, 2014), we have developed a mathematical model of plaque growth which includes the (LDL, HDL) concentrations. In the present paper, we have refined that model by including the effect of reverse cholesterol transport. By exploration-by-examples of regression of a plaque in mice, our model simulations suggest that such drugs as used for mice may also slow plaque growth in humans. We next proceeded to explore the effects of oxidative stress or antioxidant deficiency, high blood pressure and cigarette smoking as risk factors. We suggest for an individual in one of these three risk categories and with specified (LDL, HDL) concentration, how to reduce or eliminate the risk of atherosclerosis.
















Similar content being viewed by others
References
Alexander ET, Weibel GL, Joshi MR, Vedhachalam C, de la Llera-Moya M, Rothblat GH, Phillips MC, Rader DJ (2009) Macrophage reverse cholesterol transport in mice expressing ApoA-I Milano. Arterioscler Thromb Vasc Biol. 29(10):1496–501
Ambrose JA, Barua R (2004) The pathophysiology of cigarette smoking and cardiovascular disease: an update. Am Coll Cardiol 43:1731–1737
American Heart Association (2013) Heart and artery damage and high blood pressure. http://www.heart.org/Heart-and-Artery-Damage-and-High-Blood-Pressure-UCM-301823-Article.jsp
Arsenault B, Rana J, Stroes S, Despres P, Shah P (2009) Beyond low-density lipoprotein cholesterol: respective contributions of non-high-density lipoprotein cholesterol levels, triglycerides, and the total cholesterol/high-density lipoprotein cholesterol ratio to coronary heart disease risk in apparently healthy men and women. J Am Coll Cardiol 55:35–41
Azzam KM, Fessler MB (2012) Crosstalk between reverse cholesterol transport and innate immunity. Trends Endocrinol Metab 23(4):169–178
Barter P (2005) The role of HDL-cholesterol in preventing atherosclerotic disease. Eur Heart J Suppl 7:4–8
Cadenas E, Davies K (2000) Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med 29:222–230
Calvez V, Ebde A, Meunier N, Raoult A (2009) Mathematical modelling of the atherosclerotic plaque formation. CEMRACS 2008 Modell Numer Simul Complex Fluids 28:1–12
Chen WJ, Zhang M, Zhao GJ, Fu Y, Zhang DW, Zhu HB, Tang CK (2013) MicroRNA-33 in atherosclerosis etiology and pathophysiology. Atherosclerosis 227(2):201–8
Cimen M (2008) Free radical metabolism in human erythrocytes. Clin Chim Acta 390:1–11
Cohen A, Myerscough M, Thompson R (2012) Athero-protective effects of high density lipoproteins (HDL): an ODE model of the early stages of atherosclerosis, preprint
Cuchel M, Rader DJ (2006) Macrophage reverse cholesterol transport: key to the regression of atherosclerosis? Circulation 113(21):2548–2555
Cucuianu M, Coca M, Hancu N (2007) Reverse cholesterol transport and atherosclerosis. A mini review. Rom J Intern Med 45(1):17–27
Cynshi O, Kawabe Y, Tsukasa N (1998) Antiatherogenic effects of the antioxidant BO-653 in three different animal models Osamu. Proc Natl Acad Sci USA 95(17):10123–10128
Deepa T, Binu L, Sukesh A (2009) Modelling blood flow and analysis of atherosclerotic plaque rupture under G-force. Bioinform Biomed 23:1–4
de la Llera-Moya M, Drazul-Schrader D, Asztalos BF, Cuchel M, Rader DJ, Rothblat GH (2010) The ability to promote efflux via ABCA1 determines the capacity of serum specimens with similar high-density lipoprotein cholesterol to remove cholesterol from macrophages. Arterioscler Thromb Vasc Biol 30(4):796–801
Fabunmi R, Sukhova G, Sugiyama S, Libby P (1998) Expression of tissue inhibitor of metalloproteinases-3 in human atheroma and regulation in lesion-associated cells a potential protective mechanism in plaque stability. Circ Res 83:270–278
Feig JE, Parathath S, Rong JX, Mick SL, Vengrenyuk Y, Grauer L, Young SG, Fisher EA (2011a) Reversal of hyperlipidemia with a genetic switch favorably affects the content and inflammatory state of macrophages in atherosclerotic plaques. Circulation 123(9):989–998
Feig JE, Rong JX, Shamir R, Sanson M, Vengrenyuk Y, Liu J, Rayner K, Moore K, Garabedian M, Fisher EA (2011b) HDL promotes rapid atherosclerosis regression in mice and alters inflammatory properties of plaque monocyte-derived cells. Proc Natl Acad Sci 108(17):7166–7171
Francis AA, Pierce GN (2011) An integrated approach for the mechanisms responsible for atherosclerotic plaque regression. Exp Clin Cardiol 16(3):77C86
Gui T, Shimokado A, Sun Y, Akasaka T, Muragaki Y (2012) Diverse roles of macrophages in atherosclerosis: from inflammatory biology to biomarker discovery. Mediat Inflamm 2012:693083
Hansson G, Holm J, Jonasson L (1983) Detection of activated T lymphocytes in the human atherosclerotic plaque. Am J Pathol 135:169–175
Hao W, Friedman A (2014) The LDL-HDL profile determines the risk of atherosclerosis: a mathematical model. Plos One e90497
Harrington J (2000) The role of MCP-1 in atherosclerosis. Stem Cells 18:65–66
He B, Zhao S, Peng Z (2013) Effects of cigarette smoking on HDL quantity and function: implications for atherosclerosis. J Cell Biochem 114:2431–2436
Hoyert D, Xu J (2012) Deaths: preliminary data for 2011. National vital statistics reports 61
http://www.health.am/ (2004) What are LDL cholesterol particle size patterns A and B?
Hu YW, Hu YR, Zhao JY, Li SF, Ma X, Wu SG, Lu JB, Qiu YR, Sha YH, Wang YC, Gao JJ, Zheng L, Wang Q (2014) An agomir of miR-144-3p accelerates plaque formation through impairing reverse cholesterol transport and promoting pro-inflammatory cytokine production. PLoS One 9(4):e94997
Johnson J, Newby A (2009) Macrophage heterogeneity in atherosclerotic plaques. Curr Opin Lipidol 20:370–378
King I, Segal B (2005) Cutting edge: IL-12 induces CD4+CD25- T cell activation in the presence of T regulatory cells. J Immunol 175:641–645
Kosaka C, Masuda J, Shimokado K, Zen K, Yokota T, Sasaguri T, Ogata J (1992) Interferon-gamma suppresses PDGF production from THP-1 cells and blood monocyte-derived macrophages. Atherosclerosis 97:75–87
Little M, Gola A, Tzoulaki I (2009) A model of cardiovascular disease giving a plausible mechanism for the effect of fractionated low-dose Ionizing radiation exposure. PLoS Computat Biol 5:e1000539
Lovren F, Pan Y, Quan A, Singh KK, Shukla PC, Gupta N, Steer BM, Ingram AJ, Gupta M, Al-Omran M, Teoh H, Marsden PA, Verma S (2012) MicroRNA-145 targeted therapy reduces atherosclerosis. Circulation 126(11 Suppl 1):S81–90
McKay C, McKee S, Mottram N, Mulholland T Wilson S (2005) Towards a model of atherosclerosis. Strathclyde Mathematics Research Report
Moore KJ, Sheedy FJ, Fisher EA (2013) Macrophages in atherosclerosis: a dynamic balance. Nat Rev Immunol 13(10):709–721
National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) (2002) Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation 106:3143–3421
Neufeld E, Mietus-Snyder M, Beiser A, Baker A, Newburger J (1997) Passive cigarette smoking and reduced HDL cholesterol levels in children with high-risk lipid profiles. Circulation 96:1403–1407
Noguchi N, Niki E (1998) Dynamics of vitamin E action against LDL oxidation. Free Radic Res 28(6):561–572
Osterud B, Bjorklid E (2003) Role of monocytes in atherogenesis. Physiol Rev 83:1069–1112
Panousis CG, Evans G, Zuckerman SH (2011) TGF-beta increases cholesterol efflux and ABC-1 expression in macrophage-derived foam cells: opposing the effects of IFN-gamma. J Lipid Res 42(5):856–863
Pencina M, Navar-Boggan M, D’Agostino R (2014) Application of new cholesterol guidelines to a population-based sample. N Engl J Med
Powell J (1998) Vascular damage from smoking: disease mechanisms at the arterial wall. Vasc Med 3:21–28
Raines E, Ross R (1993) Smooth muscle cells and the pathogenesis of the lesions of atherosclerosis. Br Heart J 69:30–37
Ralph P, Sacco L, Benjamin E (1997) Risk factors. Stroke 28:1507–1517
Rayner KJ, Sheedy FJ, Esau CC, Hussain FN, Temel RE, Parathath S, van Gils JM, Rayner AJ, Chang AN, Suarez Y, Fernandez-Hernando C, Fisher EA, Moore KJ (2011) Antagonism of miR-33 in mice promotes reverse cholesterol transport and regression of atherosclerosis. J Clin Invest 121(7):2921–2931
Reape T, Groot P (1999) Chemokines and atherosclerosis. Atherosclerosis 147:213–225
Rodriguez J, Orbe J, Paramo J (2007) Metalloproteases, vascular remodeling, and atherothrombotic syndromes. Rev Esp Cardiol 60:959–967
Ross R, Masuda J, Raines E, Gown A, Katsuda S, Sasahara M, Malden L, Masuko H, Sato H (1990) Localization of PDGF-B protein in macrophages in all phases of atherogenesis. Science 248:1009–1012
Ryu B (2000) Low density lipoprotein (LDL), atherosclerosis and antioxidants. Biotechnol Bioprocess Eng 5:313–319
Sanson M, Distel E, Fisher EA (2013) HDL induces the expression of the M2 macrophage markers arginase 1 and Fizz-1 in a STAT6-dependent process. PLoS One 8(8):e74676
Schiopu A, Frendeus B, Jansson B, Soderberg I, Ljungcrantz I, Araya Z, Shah PK, Carlsson R, Nilsson J, Fredrikson GN (2007) Recombinant antibodies to an oxidized low-density lipoprotein epitope induce rapid regression of atherosclerosis in apobec-1(-/-)/low-density lipoprotein receptor(-/-) mice. J Am Coll Cardiol 50(24):2313–2318
Stone N, Robinson J, Lichtenstein H, Bairey M, Lloyd-Jones D (2013) 2013 ACC/AHA Guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. J Am Coll Cardiol
Tall AR, Wang N, Mucksavage P (2001) Is it time to modify the reverse cholesterol transport model? J Clin Invest 108(9):1273–1275
The top 10 causes of death. http://www.who.int/mediacentre/factsheets/fs310/en/. Accessed Dec 18, 2013
Vikstedt R, Metso J, Hakala J, Olkkonen VM, Ehnholm C, Jauhiainen M (2007) Cholesterol efflux from macrophage foam cells is enhanced by active phospholipid transfer protein through generation of two types of acceptor particles. Biochemistry 46(42):11979–11986
Wang MD, Franklin V, Marcel YL (2007a) In vivo reverse cholesterol transport from macrophages lacking ABCA1 expression is impaired. Arterioscler Thromb Vasc Biol 27(8):1837–1842
Wang X, Collins HL, Ranalletta M, Fuki IV, Billheimer JT, Rothblat GH, Tall AR, Rader DJ (2007b) Macrophage ABCA1 and ABCG1, but not SR-BI, promote macrophage reverse cholesterol transport in vivo. J Clin Invest 117(8):2216–2224
Zhang S, Ritter L, Ibragimov A (2013) Foam cell formation in atherosclerosis: HDL and macrophage reverse cholesterol transport. Discrete Contin Dyn Syst 825–835
Zhou X, He W, Huang Z, Gotto AM Jr, Hajjar DP, Han J (2008) Genetic deletion of low density lipoprotein receptor impairs sterol-induced mouse macrophage ABCA1 expression. A new SREBP1-dependent mechanism. J Biol Chem 283(4):2129–2138
Acknowledgments
This research has been supported by the Mathematical Biosciences Institute and the National Science Foundation under Grant DMS 0931642. We would like to express our thanks to Drs. Sanjay Rajagopalan and Andrei Maiseyeu at University of Maryland, Baltimore for helpful discussions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Friedman, A., Hao, W. A Mathematical Model of Atherosclerosis with Reverse Cholesterol Transport and Associated Risk Factors. Bull Math Biol 77, 758–781 (2015). https://doi.org/10.1007/s11538-014-0010-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11538-014-0010-3