Abstract
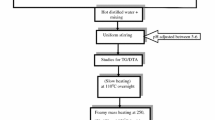
LiSmxMn2–xO4 samples were synthesized via co-precipitation technique. The structural properties of the synthesized materials were studied using X-ray diffraction analysis and it confirmed the cubic spinel structure for all the compounds. The lattice parameter of LiMn2O4 was observed to be 8.2347 Ǻ and it decreased with Sm3+ concentration, due to the shrinkage in cell volume aided by higher binding energy between Sm-O bond. The SEM micrographs were analyzed using Image processing software (Image-J) to ascertain the pore and grain properties. The microwave synthesis had been observed to control the bulk grain formation and had yielded lesser porous and nanoparticles. The particle size distributions obtained through photocross correlation laser diffraction analysis had shown that LiMn2O4 with 60 nm and Sm-doped compounds with ∼30 nm, respectively. The cyclic voltammetry studies had revealed the decrease in electrocatalytic behavior in the initial cycle for compounds doped with Sm3+ ion. The initial capacities of LiMn2O4, LiSm0.05Mn1.95O4 and LiSm0.10Mn1.90O4 substituted compounds were observed to be 134.87 mAhg−1, 132.22 mAhg−1 and 126.41 mAhg−1, respectively. The cells were simulated using 1D model namely Dualfoil5.1 program. The simulated results coincide well with the measured results. The cycle life studies reveal 93% capacity retention of samarium-0.05-doped samples when compared with 78.4% of the LiMn2O4.









Similar content being viewed by others
References
Gadjov H, Gorova M, Kotzev V, Avdeev G, Uzunova S, Kovacheva D (2004) LiMn2O4 prepared by different methods at identical thermal treatment conditions: structural, morphological and electrochemical characteristics. J Power Sources 134:110–117
Xia Y, Zhou Y, Yoshio M (1997) Capacity fading on cycling of 4 V Li/LiMn2O4cells. J Electrochem Soc 144:2593–2599
Banov B, Todorov Y, Trifonova A, Momchilov A, Manev V (1997) LiMn2–xCoxO4 cathode with enhanced cycleability. J Power Sources 68:578–581
Guohua L, Ikuta H, Uchida T, Wakihara M (1996) The spinel phases LiMyMn2–yO4(M = Co, Cr, Ni) as the cathode for rechargeable lithium batteries. J Electrochem Soc 143:178–184
Molenda J, Marzec J, Wierczek KS, Ojczyk W, Ziemnicki M, Wilk P, Molenda M, Drozdek M, Dziembaj R (2004) The effect of 3d substitutions in the manganese sublattice on the charge transport mechanism and electrochemical properties of manganese spinel. Solid State Ionics 171:215–227
Capsoni D, Bini M, Chiodell G, Massarotti V, Mustarell P, Linati L, Mozzati MC, Azzoni CB (2003) Jahn–Teller transition in Al3+ doped LiMn2O4 spinel. Solid State Commun 126:169–174
Singhal R, Das SR, Tomar MS, Ovideo O, Nieto S, Melgarejo RE, Katiyar RS (2007) Synthesis and characterization of Nd doped LiMn2O4 cathode for Li-ion rechargeable batteries. J Power Sources 164:857–861
Xie Y, Yang R, Yan L, Qi L, Dai K, He P (2007) Synthesis and electrochemical characterization of Li1.05RExCryMn2−x−yO4 spinel as cathode material for rechargeable Li-battery. J Power Sources 168:272–277
Feng C, Tang H, Zhang K, Sun J (2003) Synthesis and electrochemical characterization of nonstoichiometry spinel phase LixMn1.93Y0.02O4 for lithium ion battery applications. Mater Chem Phys 80:573–576
Peng Z-D, Hu G-R, Liu Y-X (2005) The influence on performance and structure of spinel LiMn2O4 for lithium-ion batteries by doping rare-earth Sm. J Cent South Univ Technol 12:22–32
Liu W, Kowal K, Farrington GC (1996) Electrochemical characteristics of spinel phase LiMn2O4-based cathode materials prepared by the Pechini process. J Electrochem Soc 143:3590–3600
Lu Y, Wei M, Wang Z, Evans DG, Duan X (2004) Characterization of structure and electrochemical properties of lithium manganese oxides for lithium secondary batteries hydrothermally synthesized from δ-KxMnO2. Electrochim Acta 49:2361–2367
Y-P Fu, Y-H Su, CH Lin (2004) Comparison of microwave-induced combustion and solid-state reaction for synthesis of LiMn2−xCrxO4 powders and their electrochemical properties, Solid State Ionics 166: 137–146
Ying J, Jiang C, Wan C (2004) Preparation and characterization of high-density spherical LiCoO2 cathode material for lithium ion batteries. J Power Sources 129:264–269
Ramesh PD, Bradon D (1999) Use of partially oxidized SiC particle bed for microwave sintering of low loss ceramics. Mater Sci Engg A 266:211–220
Arora P, Popov BN, White RE (1998) Electrochemical investigations of cobalt-doped LiMn2O4as cathode material for lithium-ion batteries. J Electrochem Soc 145:807–815
Kerr JA (2000) In: Lide DR (ed) CRC handbook of chemistry and physics, 81st edn. CRC Press, Boca Raton, pp 9–55
Iqbal MJ, Ahmad Z (2008) Electrical and dielectric properties of lithium manganate nanomaterials doped with rare-earth elements. J Power Sources 179:763–769
Research Services Branch NIMH & NINDS. ImageJ—Image processing and analysis in Java. Available via Web site: http://rsb.info.nih.gov/ij
Impoco G, Carrato S, Caccamo M, Tuminello L, Licitra G (2007) Quantitative analysis of cheese microstructure using SEM imagery, Communications to SIMAI Congress. 2:1827–9015
Santander N, Das SR, Majumder SB, Katiyar RS (2004) Process optimization and electrochemical properties of lithium manganate cathode for rechargeable batteries. Surf Coat Technol 177–178:60–64
Xia Y, Yoshio M (1996) An investigation of lithium ion insertion into spinel structure Li-Mn-O compounds. J Electrochem Soc 143:825–833
Liu HW, Zhang KL (2004) The synthesis and cycling behavior of LiErxMn2–xO4 for lithium-ion batteries. Mater Lett 58:3049–3051
Yang ST, Jia JH, Ding L, Zhang MC (2003) Studies of structure and cycleability of LiMn2O4 and LiNd0.01Mn1.99O4 as cathode for Li-ion batteries. Electrochim Acta 48:569–573
Doyle M, Fuller TF, Newman J (1993) Modeling of galvanostatic charge and discharge of the lithium/polymer/insertion cell. J Electrochem Soc 140:1526–1533
Doyle M, Newman J, Gozdz AS, Schmutz CN, Tarascon JM (1996) Comparison of modeling predictions with experimental data from plastic lithium ion cells. J Electrochem Soc 143:1890–1903
Fuller TF, Doyle M, Newman J (1994) Simulation and optimization of the dual lithium ion insertion cell. J Electrochem Soc 141:1–10
Stephenson DE, Hartman EM, Harb JN, Wheeler DR (2007) Modeling of particle-particle interactions in porous cathodes for lithium-ion batteries. J Electrochem Soc 154:A1146–A1155
Acknowledgement
The authors express their gratitude to the Principal and the Management of Thiagarajar College of Engineering, Madurai, India for their support, and the authors are thankful to Research Centre Imarat, Defence Research Development Organization, Hyderabad, India for their financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Balaji, S.R.K., Mutharasu, D., Shanmugan, S. et al. Influence of Sm3+ ion in structural, morphological, and electrochemical properties of LiMn2O4 synthesized by microwave calcination. Ionics 16, 351–360 (2010). https://doi.org/10.1007/s11581-009-0400-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-009-0400-y