Abstract
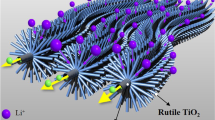
In this paper, we synthesized rutile TiO2 nanorods by hydrolysis of TiCl4 ethanolic solution in water at 50 °C. Scanning electron microscopy and transmission electron microscopy images show that the as-prepared sample was consisted of nanoflowers of about 500 nm in sizes, and each petal of nanoflowers was assembled by several nanorods. We tested the electrochemical properties of the rutile TiO2 nanorods as an anode material for lithium-ion batteries. The rutile TiO2 nanorods exhibited a large initial discharge capacity of 223 mA h g−1, and the stabilized capacity was as high as 170 mA h g−1 after 100 cycles. These improved electrochemical performances may be attributed to the shorter diffusion length for both the electron and Li+, and the large electrode–electrolyte contact area offered by the nanorods with a large specific surface area, which facilitated the lithium ions insertion and extraction.






Similar content being viewed by others
References
Whittingham MS (2004) Lithium batteries and cathode materials. Chem Rev 104:4271–4302
Wu FX, Li XH, Wang ZX, Guo HJ, Wu L, Xiong XH, Wang XJ (2011) A novel method to synthesize anatase TiO2 nanowires as an anode material for lithium-ion batteries. J Alloys Compd 509:3711–3715
Guan XF, Li LP, Li GS, Fu ZW, Zheng J, Yan TJ (2011) Hierarchical CuO hollow microspheres: controlled synthesis for enhanced lithium storage performance. J Alloys Compd 509:3367–3374
Liu H, Wexler D, Wang GX (2009) One-pot facile synthesis of iron oxide nanowires as high capacity anode materials for lithium ion batteries. J Alloys Compd 487:L24–L27
Hibino M, Abe K, Mochizuki M, Miyayama M (2004) Amorphous titanium oxide electrode for high-rate discharge and charge. J Power Sources 126:139–143
Wang Q, Wen ZH, Li JH (2006) Solvent-controlled synthesis and electrochemical lithium storage of one-dimensional TiO2 nanostructures. Inorg Chem 45:6944–6949
Gao XP, Zhu HY, Pan GL, Ye SH, Lan Y, Wu F, Song DY (2004) Preparation and electrochemical characterization of anatase nanorods for lithium-inserting electrode material. J Phys Chem B 108:2868–2872
Qiao H, Wang YW, Xiao LF, Zhang LZ (2008) High lithium electroactivity of hierarchical porous rutile TiO2 nanorod microspheres. Electrochem Commun 10:1280–1283
Wang YD, Chen T, Mu QY (2011) Electrochemical performance of W-doped anatase TiO2 nanoparticles as an electrode material for lithium-ion batteries. J Mater Chem 21:6006–6013
Jung HG, Yoon CS, Prakash J (2009) Mesoporous anatse TiO2 with high surface area and controllable pore size by F(−)- ion doping: applications for high-power Li-ion battery anode. J Phys Chem C 113:21258–21263
Das SK, Bhattacharyya AJ (2009) High lithium storage in mixed crystallographic phase nanotubes of titania and carbon-titania. J Phys Chem C 113:17367–17371
Mancini M, Kubiak P, Geserick J, Marassi R, Hüsing N, Wohlfahrt-Mehrens M (2009) Mesoporous anatase TiO2 composite electrodes: Electrochemical characterization and high rate performances. J Power Sources 189:585–589
Armstrong AR, Armstrong G, Canales J, Garcia R, Bruce PG (2005) Lithium-ion intercalation into TiO2–B nanowires. Adv Mater 17:862–865
Wagemaker M, Kentgens APM, Mulder FM (2002) Equilibrium lithium transport between nanocrystalline phases in intercalated TiO2 anatase. Nature 418:397–399
Wagemaker M, Van de Krol R, Kentgens APM, Well AA, Mulder FM (2001) Two phase morphology limits lithium diffusion in TiO2 (anatase): A7Li MAS NMR study. J Am Chem Soc 123:11454–11461
Sudant G, Baudrin E, Larcher D, Tarascon JM (2005) Electrochemical lithium reactivity with nanotextured anatase-type TiO2. J Mater Chem 15:1263–1269
Baudrin E, Cassaignon S, Koelsch M, Jolivet JP, Dupont L, Tarascon JM (2007) Structural evolution during the reaction of Li with nano-sized rutile type TiO2 at room temperature. Electrochem Commun 9:337–342
Anji Reddy M, Satya Kishore M, Pralong V, Caignaert V, Varadaraju UV, Raveau B (2006) Room temperature synthesis and Li insertion into nanocrystalline rutile TiO2. Electrochem Commun 8:1299–1303
Kubiak P, Pfanzelta M, Geserick J, Hörmann U, Hüsing N, Kaiser U, Wohlfahrt-Mehrens M (2009) Electrochemical evaluation of rutile TiO2 nanoparticles as negative electrode for Li-ion batteries. J Power Sources 194:1099–1104
Vijayakumar M, Kerisit S, Wang CM, Nie ZM, Rosso KM, Yang ZG, Graff G, Liu J, Hu JZ (2009) Effects of chemical lithium insertion into rutile TiO2 nanorods. J Phys Chem C 113:14567–14574
Wang DH, Choi D, Yang ZG, Viswanathan VV, Nie ZM, Wang CM, Song YJ, Zhang JG, Liu J (2008) Synthesis and Li-ion insertion properties of highly crystalline mesoporous rutile TiO2. Chem Mater 20:3435–3442
Borghols WJH, Lutzenkirchen-Hecht D, Haake U (2010) Lithium storage in amorphous TiO2 nanoparticles. J Electrochem Soc 157:A582–A588
Milne NA, Skyllas-Kazacos M, Luca V (2009) Crystallite size dependence of lithium intercalation in nanocrystalline rutile. J Phys Chem C 113:12983–12995
Bao SJ, Bao QL, Li CM, Dong ZL (2007) Novel porous anatase TiO2 nanorods and their high lithium electroactivity. Electrochem Commun 9:1233–1238
Choi MG, Lee YG, Song SW (2010) Anode properties of titanium oxide nanotude and graphite composites for lithium-ion batteries. J Power Sources 195:8289–8296
Choi MG, Lee YG, Song SW (2010) Lithium-ion battery anode properties of TiO2 nanotubes prepared by the hydrothermal synthesis of mixed (anatase and rutile) particles. Electrochim Acta 55:5975–5983
Xu JW, Jia CH, Cao B, Zhang WF (2007) Electrochemical properties of anatase TiO2 nanotubes as an anode material for lithium-ion batteries. Electrochim Acta 52:8044–8047
Fang D, Huang KL, Liu SQ (2008) Electrochemical properties of ordered TiO2 annotube loaded with Ag nano-particels for lithium anode material. J Alloys Compd 464:L5–L9
Armstrong AR, Armstrong G, Canales J, Bruce PG (2005) TiO2-B nanowires as negative electrodes for rechargeable lithium batteries. J Power Sources 146:501–506
Sing KSW, Everett DH, Haul RAW, Moscou L, Pierotti RA, Rouquerol J, Siemieniewska T (1985) Reporting physisorption data for gas/solid systems. Pure & Appl Chem 57:603–619
Chen JS, Lou XW (2010) The superior lithium storage capabilities of ultra-fine rutile TiO2 nanoparticles. J Power Sources 195:2905–2908
Hu YS, Kienle L, Guo YG, Maier J (2006) High lithium electroactivity of nanometer-sized rutile TiO2. Adv Mater 18:1421–1426
Acknowledgments
This work was financially supported by the Fundamental Research Funds for the Central Universities (JUSRP11102, JUSRP20903, and JUSRP31101), the National Natural Science Foundation of China (51006046), the Natural Science Fundation of Jiangsu Province (BK2010140), and the Research Fund for the Doctoral Program of Higher Education of China (200802951011 and 20090093110004).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qiao, H., Luo, Q., Wei, Q. et al. Electrochemical properties of rutile TiO2 nanorods as anode material for lithium-ion batteries. Ionics 18, 667–672 (2012). https://doi.org/10.1007/s11581-012-0672-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-012-0672-5