Abstract
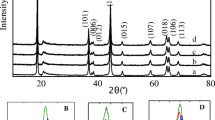
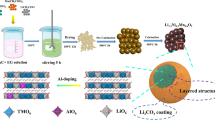
Layered cathode material Li1.2Ni0.2Mn0.6O2 has been synthesized using a coprecipitation method and coated by MnO2 with varying amounts (1, 3, 5, and 9 wt%). The physical properties and electrochemical performances of the materials are characterized by XRD, SEM, charge/discharge tests, cycle life, and rate capability tests. XRD patterns show that the pristine and coated Li1.2Ni0.2Mn0.6O2 powders exhibit layered structure. The discharge capacities and coulombic efficiencies of Li1.2Ni0.2Mn0.6O2 in the first cycle have been improved and increase with the increasing content of coated MnO2. The 9 wt% MnO2-coated Li1.2Ni0.2Mn0.6O2 delivers 287 mAhg−1 for the first discharge capacity and 86.7 % for the first coulombic efficiency compared with 228 mAhg−1 and 65.9 % for pristine Li1.2Ni0.2Mn0.6O2. However, the 5 wt% MnO2-coated Li1.2Ni0.2Mn0.6O2 shows the best capacity retention (99.9 % for 50 cycles) and rate capability (88.6 mAhg−1 at 10 C), while the pristine Li1.2Ni0.2Mn0.6O2 only shows 91.5 % for 50 cycles and 25.3 mAhg−1 at 10 C. The charge/discharge curves and differential capacity vs. voltage (dQ/dV) curves show that the coated MnO2 reacts with Li+ during the charge and discharge process, which is responsible for higher discharge capacity after coating. Electrochemical impedance spectroscopy results show that the R ct of Li1.2Ni0.2Mn0.6O2 electrode decreases after coating, which is responsible for superior rate capability.








Similar content being viewed by others
References
Wang CC, Jarvis KA, Ferreira PJ, Manthiram A (2013) Chem Mater 25:3267
Wu F, Lu HQ, Su YF, Li N, Bao LY, Chen S (2010) J Appl Electrochem 40:783
Wen JG, Bareno J, Lei CH, Kang SH, Balasubramanian M, Petrov I, Abraham DP (2011) Solid State Ionics 182:98
Wang J, Qiu B, Cao HL, Xia YG, Liu ZP (2012) J Power Sources 218:128
Song BH, Liu ZW, Lai MO, Lu L (2012) Phys Chem Chem Phys 14:12875
Zheng JM, Zhang ZR, Wu XB, Dong ZX, Zhu Z, Yang Y (2008) J Electrochem Soc 155:A775
He W, Qian JF, Cao YL, Ai XP, Yang HX (2012) RSC Adv 2:3423
Wu Y, Manthiram A (2009) Solid State Ionics 180:50
Kang SH, Thackeray MM (2009) Electrochem Commun 11:748
Shi SJ, Tu JP, Tang YY, Liu XY, Zhang YQ, Wang XL, Gu CD (2013) Electrochim Acta 88:671
Liu B, Zhang Q, He SC, Satob YC, Zheng JW, Li DC (2011) Electrochim Acta 56:6748
Bach S, Pereira-Ramos JP, Willmann P (2011) Electrochim Acta 56(27):10016
Jouanneau S, Sarciaux S, Salle A, Guyomard D (2001) Solid State Ionics 140:223
Liu YJ, Liu SB, Wang YP, Chen L, Chen XH (2013) J Power Sources 222:455
F.W, N. Li, Y. F. Su, H. Q. Lu, L. J. Zhang, R. An, Z. Wang, L. Y. Bao, S. (2012) Chen. J. Mater. Chem. 22: 1489.
Yabuuchi N, Makimura Y, Ohzuku T (2007) J Electrochem Soc 154:A314
Li GR, Feng X, Ding Y, Ye SH, Gao XP (2012) Electrochim Acta 78:308
Acknowledgments
The authors gratefully acknowledge the National Natural Science Foundation of China (51304081) and Postdoctoral Foundation of China (2012M511211).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, Y., Gao, Y., Wang, Q. et al. Influence of coated MnO2 content on the electrochemical performance of Li1.2Ni0.2Mn0.6O2 cathodes. Ionics 20, 825–831 (2014). https://doi.org/10.1007/s11581-013-1048-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-013-1048-1