Abstract
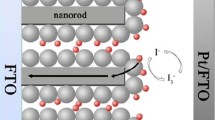
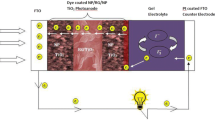
The novel TiO2 nanopartilces/nanowires (TNPWs) composite with ZrO2 nanoparticles (ZNPs) shell-coated photoanodes were prepared to fabricate high-performance dye-sensitized solar cell (DSSC) based on different types of electrolytes. Hafnium oxide (HfO2) is a new and efficient blocking layer material applied over the TNPWs-ZNPs core-shell photoanode film. TiO2 nanoparticles (TNPs) and TiO2 nanowires (TNWs) were characterized by X-ray diffractometer (XRD), scanning electron microscopy (SEM), and transmission electron microscopy (TEM). DSSCs were fabricated using the novel photoanodes with an organic sensitizer D149 dye and different types of electrolytes namely liquid electrolyte, ionic liquid electrolyte, solid-state electrolyte, and quasi-solid-state electrolyte. The DSSC-4 made through the novel core-shell photoanode using quasi-solid-state electrolyte showed better photocurrent efficiency (PCE) as compared to the other DSSCs. It has such photocurrent-voltage characteristics: short circuit photocurrent (Jsc) = 19 mA/cm2, the open circuit voltage (Voc) = 650 mV, fill factor (FF) = 65 %, and PCE (η) = 8.03 %. The improved performance of DSSC-4 is ascribed to the core-shell with blocking layer photoanode could increased electron transport and suppressed recombination of charge carriers at the TNPWs-ZNPs/dye/electrolyte interface.










Similar content being viewed by others
References
Adachi M, Murata Y, Takao J, Jiu J, Sakamoto M, Wang FM (2004) J Am Chem Soc 126:14943–14949
Chae WS, Lee SW, Kim YR (2005) Chem Mater 17:3072–3074
O’Regan B, Gratzel M (1991) Nature 353:737–739
Zhang Q, Cao G (2011) Nano Today 6:91–109
Chappel S, Chen SG, Zaban A (2002) Langmuir 18:3336–3342
Kay A, Gratzel M (2002) Chem Mater 14:2930–2935
Hod I, Shalom M, Tachan Z, Ruhle S, Zaban A (2010) J Phys Chem C 114:10015–10018
Palomares E, Clifford JN, Haque SA, Lutz T, Durrant JR (2002) J Am Chem Soc 125:475–482
Bisquert J, Zaban A, Greenshtein M, Mora-Sero I (2004) J Am Chem Soc 126:13550–13559
Park K, Jin E, Gu H, Yoon S, Han E, Yun J (2010) Appl Phys Lett 97:023302–023304
Ramasamy P, Kang M, Cha H, Kim J (2013) Mater Res Bull 48:79–83
Lai Y, Chiu C, Chen J, Wang C, Lin J, Lin K, Ho K (2009) Sol Energy Mater Sol Cells 93:1860–1864
Lee CH, Rhee SW, Choi HW (2012) Nanoscale Res Lett 7:48–52
Gillet M, Delamare R, Gillet E (2005) J Cryst Growth 279:93–99
Kim JY, Lee S, Noh JH, Jung HS, Hong KS (2009) J Electroceram 23:422–425
Bisquert J (2002) J Phys Chem B 106:325
Tan B, Wu Y (2006) J Phys Chem B 110:15932–15938
Wu JJ, Chen GR, Lu CC, Wu WT, Chen JS (2008) Nanotechnology 19:105702–105708
Adachi M, Sakamoto M, Jiu J, Ogata Y, Isoda S (2006) J Phys Chem B 110:13872–13880
Watson DF, Meyer GJ (2004) Coord Chem Rev 248:1391–1406
Fujihara K, Kumar A, Jose R, Ramakrishna S, Uchida S (2007) Nanotechnology 18:365709
Baxter JB, Aydil ES (2006) Sol Energy Mater Sol Cells 90:607–622
Qi L, Liu Y, Li C (2010) Appl Surf Sci 257:1660–1665
Hagfeldt A, Boschloo G, Sun L, Kloo L, Pettersson H (2010) Chem Rev 110:6595–6663
Kanmani SS, Ramachandran (2012) Renew Energy 43:149–156
Acknowledgments
The authors are thankful to the authorities of Annamalai University for providing all necessary facilities to carry out the present work successfully. We also thank the anonymous referees who contributed significantly to improving the contents of the manuscript.
Conflict of interest
The authors declare that they have no conflicts of interest concerning this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Manoharan, K., Joby, N.G. & Venkatachalam, P. A novel TiO2 nanoparticles/nanowires composite core with ZrO2 nanoparticles shell coating photoanode for high-performance dye-sensitized solar cell based on different electrolytes. Ionics 20, 887–896 (2014). https://doi.org/10.1007/s11581-013-1050-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-013-1050-7