Abstract
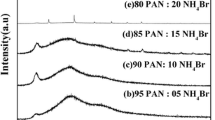
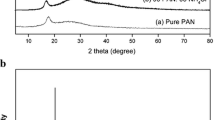
Blending of polymers is one of the most useful methods for modulating the conductivity of solid polymer electrolytes. Blend polymer electrolytes have been prepared with polyvinyl alcohol (PVA)-polyacrylonitrile (PAN) blend doped with ammonium thiocyanate with different concentrations by solution casting technique, using dimethyl formamide (DMF) as the solvent. The prepared electrolytes are characterized by X-ray diffraction (XRD), Fourier transform infrared (FTIR), differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), nuclear magnetic resonance (NMR), ultraviolet (UV), and ac impedance measurement techniques. The increase in amorphous nature of the blend polymer electrolyte by the addition of salt is confirmed by XRD analysis. The complex formation between the polymers and the salt has been confirmed by FTIR analysis. The thermal behavior has been examined using DSC and TGA. The maximum conductivity has been found to be 2.4 × 10−3 S cm−1 for 92.5PVA/7.5PAN/25 % NH4SCN sample at room temperature. The temperature dependence of conductivity has been studied with the help of Arrhenius plot, and the activation energies are calculated. The proton conductivity is confirmed by dc polarization measurement technique. 1H NMR studies reveal the presence of protons in the sample. A proton battery is constructed with the highest conducting sample, and its open circuit voltage is measured to be 1.2 V













Similar content being viewed by others
References
Subba Reddy CV, Sharmanad AK, NarasimhaRao VVR (2004) Characterization of a solid state battery based on polyblend of (PVP + PVA + KBrO3) electrolyte. Ionics 10:142–147
Varnell DF, Coleman MM (1981) FT I.R. studies of polymer blends: V. Further observations on polyester-poly(vinyl chloride) blends. Polymer 22:1324–1328
Garton A, Aubin M, Prudhomme RE (1983) FTIR of polycaprolactone/poly(vinylidene chloride-coacrylonitrile) miscible blends. J Polym Sci Polym Lett Edn 21:45–47
Baskaran R, Selvasekarapandian S, Kuwata N, Kawamwa J, Hattori T (2006) Conductivity and thermal studies of blend polymer electrolytes based on PVAc–PMMA. Solid State Ionics 177:2679
Rajeswari N, Selvasekarapandian S, Karthikeyan S, Prabu M, Hiran Kumar G, Nithya H, Sanjeeviraja C (2011) Conductivity and dielectric properties of polyvinyl alcohol–polyvinyl pyrrolidone poly blend film using non-aqueous medium. J Non-Cryst Solids 357:3751
Sivadevi S, Selvasekarapandian S, Karthikeyan S, Vijaya N, Kingslin Mary Genova F, Sanjeeviraja C (2013) Structural and AC impedance analysis of blend polymer electrolyte based on PVA and PAN. International Journal of Scientific Research 2(10)
Subramanian A, Kalyana Sundaram NT, Vijaya Kumar G, Vasudevan T (2006) Effect of salt concentration in (PVA-PAN) based plasticized polymer blend electrolyte for Li ion battery application. Ionics 12:175
Sivadevi S, Selvasekarapandian S, Karthikeyan S, Vijaya N, Kingslin FMG, Sanjeeviraja C, Nithya H, Junichi Kawamura I (2013) Proton-conducting polymer electrolyte based on PVA-PAN blend polymer doped with NH4NO3. Int J Electroact Mater 1:64–70
Hirankumar G, Selvasekarapandian S, Bhuvaneswwari MS, Baskaran R, Vijayakumar M (2006) Ag + ion transport studies in a polyvinyl alcohol-based polymer electrolyte system. J Solid State Electrochem 10:193
Hodge RM, Edward GH, Simon GP (1996) Water absorption and states of water in semicrystalline poly(vinyl alcohol) films. Polymer 37:1371
Xu M, Eyring EM, Petrueei S (1995) Molecular dynamics and infrared spectra of NaSCN dissolved in the solvent macrocycle 15-crown-5 and polyethylene oxide dimethyl ether-250. J Phys Chem 99:14589
Radha KP, Selvasekarapandian S, Karthikeyan S, Hema M, Sanjeeviraja C (2013) Ionics. Issue 760
Hirankumar G, Selvasekarapandian S, Bhuvaneswari MS, Baskaran R, Vijayakumar M (2004) AC impedance studies on proton conducting polymer electrolyte complexes (PVA + CH3 COONH4). Ionics 10:135
Chabanel M, Wang Z (1984) Vibrational study of ionic association in aprotic solvents. 6. Dimerization of lithium, sodium, and potassium isothiocyanate ion pairs in tetrahydrofuran and in 1,3-dioxolane. J Phys Chem 88:1441
Zhang H, Xuan X, Wang J, Wamg H (2003) FT-IR investigation of ion association in PEO–MSCN (M = Na, K) polymer electrolytes. Solid State Ionics 164:73–79
Irish DE, Tang SY, Talts H, Petrucci S (1979) Raman, infrared, and ultrasonic relaxation studies of some sodium and lithium salts in dimethylacetamide. J Phys Chem 83:3268
Saar D, Petrucci P (1986) Infrared and ultrasonic spectra of sodium thiocyanate and lithium thiocyanate in tetrahydrofuran. J Phys Chem 90:3326
Bacelon P, Corset J, De Loze C (1980) Triple ion formation in solutions of alkaline sulfocyanides. J Solut Chem 9:129
Hiran Kumar G (2005) Characterization of solid polymer electrolyte based on poly(vinyl alcohol). Bharathiar University, Coimbatore
Selvasekarapandian S, Hema M, Kawamura J, Kamishima O, Baskaran R (2010) Characterization of PVA–NH4NO3 polymer electrolyte and its application in rechargeable proton battery. J Phys Soc Jpn Suppl A 79:163–168
Ramesh S, Arof AK (2001) Structural, thermal and electrochemical cell characteristics of poly(vinyl chloride) based polymer electrolytes. J Power Sources 99:41
Samsudin AS, Lai HM, Isa MIN (2014) Biopolymer materials based carboxymethyl cellulose as a proton conducting biopolymer electrolyte for application in rechargeable proton battery. Electrochim Acta 129:1–13
Ma X-H, Xu Z-L, Liu Y, Sun D (2010) Preparation and characterization of PFSA–PVA–SiO2/PVA/PAN difunctional hollow fiber composite membranes. J Membr Sci 360:3
Huang X, Ma X, Gao J, Tan B, Yang K, Wang G, Deng Z (2012) Preparation, characterization, conductivity studies of novel solid polymer electrolytes based on blend of poly (AN-co-VEC) and EVA. Solid State Ionics 215:7–15
Hema M, Selvasekarapandian S, Hirankumar G (2007) Vibrational and impedance spectroscopic analysis of poly(vinyl alcohol)-based solid polymer electrolytes. Ionics 13:483
Kadir MFZ, Majid SR, Arof AK (2010) Plasticized chitosan-PVA blend based proton battery. Electrochim Acta 55:1475–1482
Ramesh S, Arof AK (2001) Ionic conductivity studies of plasticized poly(vinyl chloride) polymer electrolytes. Mater Sci Eng B 85:11–15
Vijaya N, Selvasekarapandian S, Hirankumar G, Karthikeyan S, Nithya H, Ramya CS, Prabu M (2012) Structural, vibrational, thermal, and conductivity studies on proton-conducting polymer electrolyte based on poly(N-vinylpyrrolidone). Ionics 18:91–99
Mishra R, Baskaran N, Ramakrishnan PA, Rao KJ (1998) Lithium ion conduction in extreme polymer in salt regime. Solid State Ionics 112:261–273
Cameron GG, Ingram MD, Sorrie GA (1986) Ionic conductivity in liquid polymeric electrolytes. IJ Electroanal Chem 198:205
Wagner JB, Wagner CJ (1957) Electrical conductivity measurements on cuprous halides. Chem Rev 26:1597
Singh K, Tiwari RU, Deshpande VK (1993) Performance of a solid-state battery with a proton-conducting electrolyte. J Power Sources 46:65
De Bruin HJ, Badwal SPS (1978) Electrode kinetics at the palladium/ceramic oxide electrolyte interface. J Am Ceram Soc 14:20
Kamlesh Pandey N, Lakshmi S, Chandra S (1998) A rechargeable solid-state proton battery with an intercalating cathode and an anode containing a hydrogen-storage material. J Power Sources 76:116
Rajeswari N, Selvasekarapandian S, Sanjeeviraja C, Kawamura J, Asath Bahadur S (2014) A study on polymer blend electrolyte based on PVA/PVP with proton salt. Polym Bull 71:1061–1080
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sivadevi, S., Selvasekarapandian, S., Karthikeyan, S. et al. Proton-conducting polymer electrolyte based on PVA-PAN blend doped with ammonium thiocyanate. Ionics 21, 1017–1029 (2015). https://doi.org/10.1007/s11581-014-1259-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-014-1259-0