Abstract
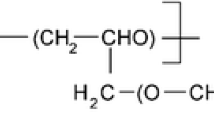
We investigated the structural and thermal properties of a gel polymer electrolyte prepared with poly(ethylene oxide-co-2-(2-methoxyethoxy)ethyl glycidyl ether) (P(EO/EM) matrix, γ-butyrolactone (GBL), and LiI/I2. Nuclear magnetic resonance spectroscopy measurements were carried out in different temperatures for the aforementioned electrolyte composition in order to understand the interaction between the components of the gel. Within the studied temperature range, the overall Li ion mobility depends on the contribution of the Li ion mobility in GBL molecules and in the polymer matrix. Small angle X-ray scattering (SAXS) measurements of the gel electrolyte indicated an order periodicity of 7–8 nm, which provides evidence for an effective miscibility of GBL and P(EO/EM), since no structural changes were observed by SAXS data (the systems are stable within the studied temperature range). Indeed, this result corroborates Nuclear Magnetic Resonance Spectroscopy (NMR) data, since at room temperature, it is not possible to distinguish magnetic interactions between P(EO-EM) and GBL molecules, indicating the existence of a single phase. Thermogravimetric analysis shows that the gel electrolyte meets thermal stability requirements for application in photoelectrochemical devices. The performance of dye-sensitized solar cells (DSSC) based on this polymer electrolyte with different GBL concentrations was monitored for 30 days. Device with electrolyte containing 30 wt.% of GBL gave the most promising durability results in this study; the performance of this device decreased by less than 10 % in the course of 30 days.









Similar content being viewed by others
References
Manfredi N, Bianchi A, Causin V, Ruffo R, Simonutti R, Abbotto A (2014) Electrolytes for quasi solid-state dye-sensitized solar cells based on block copolymers. J Polym Sci A Polym Chem 52:719–727
Avellaneda CO, Gonçalves AS, Benedetti JE, Nogueira AF (2010) Preparation and characterization of core/shell electrodes for application in gel/electrolyte/based dye/sensitized solar cells. Electrochim Acta 55:1468–1474
O’Regan B, Grätzel M (1991) A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 353:737–740
Subramania A, Vijayakumar E, Sivasankar N, Sathiya Priya AR, Jin Kim K (2013) Effect of different compositions of ethylene carbonate and propylene carbonate containing iodide/triiodide redox electrolyte on the photovoltaic performance of DSSC. Ionics 19:1649–1653
Benedetti JE, Paoli MA, Nogueira AF (2008) Enhancement of photocurrent generation and open circuit voltage in dye-sensitized TiO2 solar cells using Li+ trapping species in the gel electrolyte. Chem Commun 9:288–692
Qinghua L, Chen X, Tang Q, Cai H, Qin Y, He B, Li M, Jin S, Liu Z (2014) Enhanced photovoltaic performances of quasi-solid-state dye-sensitized solar cells using a novel conducting gel electrolyte. J Power Sources 248:923–930
Ghavre M, Byrne O, Altes L, Surolia PK, Spulak M, Quilty B, Thampi KR, Gathergood N (2014) Low toxicity functionalised imidazolium salts for task specific ionic liquid electrolytes in dye-sensitised solar cells: a step towards less hazardous energy production. Green Chem 16:2252–2265
Li Q, Tang Q, Du N, Qin Y, Xiao J, He B, Chen H, Lei C (2014) Employment of ionic liquid-imbibed polymer gel electrolyte for efficient quasi-solid-state dye-sensitized solar cells. J Power Sources 248:816–821
Senadeera GKR, Kitamura T, Wada Y, Yanagida S (2006) Enhanced photoresponses of polypyrrole on surface modifiedTiO2 with self-assembled monolayers. J Photochem Photobiol A 184:234–239
Bandara J, Weerasinghe H (2006) Solid-state dye-sensitized solar cell with p-type NiO as a hole collector. Sol Energy Mater Sol 85:385–390
White RC, Benedetti JE, Gonçalves AS, Romão W, Vaz BG, Eberlin MN, Correia CRD, Paoli MA, Nogueira AF (2011) Synthesis, characterization and introduction of a new ion-coordinating ruthenium sensitizer dye in quasi-solid state TiO2 solar cells. J Photochem Photobiol A 222:185–191
Freitas JN, Longo C, Nogueira AF, De Paoli M-A (2008) Solar module using dye-sensitized solar cells with a polymer electrolyte. Sol Energy Mater Sol Cells 92:1110–1114
Fenton DE, Parker JM, Wright PV (1973) Complexes of alkali metal ions with poly(ethylene oxide). Polymer 14(1973):589
Nogueira AF, Longo C, De Paoli MA (2004) Polymers in dye sensitized solar cells: overview and perspectives. Coord Chem Rev 248:1455–1468
Rajasudha G, Jayan LM, Lakshmi DD, Thangadurai P, Boukos N, Narayanan V, Stephen A (2012) Polyindole–CuO composite polymer electrolyte containing LiClO4 for lithium ion polymer batteries. Polym Bull 68:181–196
Yu H, Wu J, Fan L, Xu K, Zhong X, Lin Y, Lin J (2011) Improvement of the performance for quasi-solid-state supercapacitor by using PVA–KOH–KI polymer gel electrolyte. Electrochim Acta 56:6881–6886
Lee KT, Lee JF, Wu NL (2009) Electrochemical characterizations on MnO2 supercapacitors with potassium polyacrylate and potassium polyacrylate-co-polyacrylamide gel polymer electrolytes. Electrochim Acta 54:6148–6153
Carol P, Ramakrishnan P, John B, Cheruvally G (2011) Preparation and characterization of electrospun poly(acrylonitrile) fibrous membrane based gel polymer electrolytes for lithium-ion batteries. J Power Sources 196:10156–10162
Liu LL, Li ZH, Xia QL, Xiao QZ, Lei GT, Zhou XD, Zhou XD (2012) Electrochemical study of P(VDF-HFP)/PMMA blended polymer electrolyte with high-temperature stability for polymer lithium secondary batteries. Ionics 18:275–281
Al-Kahlout A, Diogo D, Avellaneda CO, Leite ER, Aegerter MA, Pawlicka A (2010) Gelatin-based protonic electrolyte for electrochromic windows. Ionics 16:13–19
Alias SS, Mohamad AA (2013) Effect of NH4I and I2 concentration on agar gel polymer electrolyte properties for a dye-sensitized solar cell. Ionics 19:1185–1194
Huang KC, Chen PY, Vittal R, Ho KC (2011) Enhanced performance of a quasi-solid-state dye-sensitized solar cell with aluminum nitride in its gel polymer electrolyte. Energ Mater Sol Cells 95:1990–1995
Freitas FS, Freitas JN, Ito BI, De Paoli MA, Nogueira AF (2009) Electrochemical and structural characterization of polymer gel electrolytes based on a PEO copolymer and an imidazolium-based ionic liquid for dye-sensitized solar cells. Appl Mater Interfaces 1:2870–2877
Freitas JN, Gonçalves AS, Durrant JR, De Paoli MA, Nogueira AF (2008) The role of gel electrolyte composition in the kinetics and performance of dye-sensitized solar cells. Electrochim Acta 53:7166–7172
Benedetti JE, Gonçalves AS, Formiga ALB, Paoli MA, Durrant JR (2010) A polymer gel electrolyte composed of a poly(ethylene oxide) copolymer and the influence of its composition on the dynamics and performance of dye-sensitized solar cells. J Power Sources 195:1246–1255
Kim CS, Oh SM (2001) Performance of gel-type polymer electrolytes according to the affinity between polymer matrix and plasticizing solvent molecules. Electrochim Acta 46:1323–1331
Kang Y, Cheong K, Noh KA, Lee C, Seung DY (2003) A study of cross-linked PEO gel polymer electrolytes using bisphenol A ethoxylate diacrylate: ionic conductivity and mechanical properties. J Power Sources 119–121:432–437
Han HW, Bach U, Cheng YB, Caruso RA (2007) Increased nanopore filling: effect on monolithic all-solid-state dye-sensitized solar cells. Appl Phys Lett 90:213510
Wu JH, Lan Z, Lin JM, Huang ML, Hao SC, Sato T, Yin S (2007) A novel thermosetting gel electrolyte for stable quasi-solid-state dye-sensitized solar cells. Adv Mater 19:4006–4011
Huanga B, Wang Z, Chen L, Xue R, Wang F (1996) The mechanism of lithium ion transport in polyacrylonitrile-based polymer electrolytes. Solid State Ionics 91:279–284
Panero S, Scrosati B, Greenbaum SG (1992) Complex impedance measurements on Nafion. Electrochim Acta 37:1533–1538
Avellaneda CO, Vieira DF, Al-Kahlout A, Heusinga S, Leite ER, Pawlicka A, Aegerte MA (2008) All solid-state electrochromic devices with gelatin-based electrolyte. Sol Energy Mat Sol Cells 92:228–233
Guo R, Wang H, Peng C, Shen M, Zheng L, Zhang G, Shi X (2011) Enhanced X-ray attenuation property of dendrimer-entrapped gold nanoparticles complexed with diatrizoic acid. J Mater Chem 21:5120–5127
Kang M-S, Kim JH, Won J, Kang YS, Sol. Energ (2006) Phase behavior of lithium perchlorate-doped poly(styrene-b -isoprene-b-ethylene oxide) triblock copolymers. Mat Sol Cells 183:15
Epps TH, Bailey TS, Pham HD, Bates FS (2002) Phase behavior of lithium perchlorate-doped poly(styrene-b -isoprene-b-ethylene oxide) triblock copolymers. Chem Mater 14:1706–1714
Roh DK, Park TJ, Ahn SH, Ahn H, Ryu DY, Kim JH (2010) Amphiphilic poly(vinyl chloride)-g-poly(oxyethylene methacrylate) graft polymer electrolytes: interactions, nanostructures and applications to dye-sensitized solar cells. Electrochim Acta 55:4976–4981
A Guinier, G Fournet (1955) Small-angle scattering of X-rays (structure of matter series) Wiley, New York p 126
Sommeling PM, Späth M, Smit HJP, Bakker NJ, Kroon JM (2004) Long-term stability testing of dye-sensitized solar cells. J Photoch Photobiol A 164:137–144
Hinsch A, Kroon JM, Kern R, Uhlendorf I, Holzbock J, Meyer A (2001) Long-term stability of dye-sensitised solar cells. Prog Photovolt Res Appl 9:425–438
Nogueira AF, Spinacé MAS, Gazotti WA, Girotto EM, De Paoli MA (2001) Poly(ethylene oxide-co-epichlorohydrinr)/NaI: a promising polymer electrolyte for photoelectrochemical cells. Solid State Ionics 140:327–335
Costa L, Gad AM, Camino G, Cameron GG (1992) Thermal and thermooxidative degradation of poly(ethy1eneoxide)-metal salt complexes. Macromolecules 25:5512–5518
Chen D, Zhang Q, Wang G, Zhang H, Li J (2007) A novel composite polymer electrolyte containing room-temperature ionic liquids and heteropolyacids for dye-sensitized solar cells. Sci China Chem 9:2755–2759
Huang J, Liu X, Hang X, Yu Z, Xu T, Qiu W, Kang Y, Cheong K, Noh KA, Lee C, Seung DY (2009) Study on-butyrolactone for LiBOB-based electrolyte. J Power Sources 189:458–461
Acknowledgments
The authors acknowledge FAPESP (fellowships 2011/080304-6, 08/51001-9 and 11/50933-8) and CNPq for the financial support, Daiso Co. Ltd. Osaka (Japan) for providing kindly the copolymer, and Keru Kamitha for the English revision of this manuscript. We also acknowledge the Brazilian National Nanotechnology Laboratory for the use of the SAXS line and FE-SEM facilities.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOC 248 kb)
Rights and permissions
About this article
Cite this article
Benedetti, J.E., Freitas, F.S., Fernandes, F.C. et al. Investigation of the structural properties of poly(ethylene oxide) copolymer as gel polymer electrolyte and durability test in dye-sensitized solar cells. Ionics 21, 1771–1780 (2015). https://doi.org/10.1007/s11581-014-1318-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-014-1318-6