Abstract
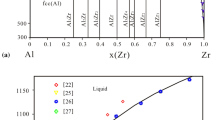
The Al2O3-SrO binary system has been studied using the CALPHAD technique in this paper. The modeling of Al2O3 in the liquid phase is modified from the traditional formula with the liquid phase represented by the ionic two-sublattice model as (Al3+, Sr2+) P (\({\hbox{AlO}_{2}^{1-}}\), O2−) Q . Based on the measured phase equilibrium data and experimental thermodynamic properties, a set of thermodynamic functions has been optimized using an interactive computer-assisted analysis. The calculated results are compared with experimental data. A comparison between this system and similar systems is also given.


Similar content being viewed by others
References
Ohta M., Maruyama M., Hayakawa T., Nishijo T. (2000) Role of Dopant on Long-lasting Phosphor of Strontium Aluminate. J. Ceram. Soc. Jpn 108(3):284-289
Mao H., Selleby M., Sundman B. (2004) A Reevaluation of the Liquid Phases in the CaO-Al2O3 and MgO-Al2O3 Systems. Calphad 28(3):307-312
Ye X., Zhuang W., Deng C., Yuan W., Qiao Z. (2006) Thermodynamic Investigation on the Al2O3-BaO Binary System. Calphad 30 (3):349-353
Lagerqvist L., Wallmark S., Westgren A. (1937) X-ray study of the systems CaO-Al2O3 and MgO-Al2O3. Z. Anorg. Allgem. Chem. 234:1-16
Jander W., Kreiger A. (1937) Formation of Strontium Aluminates from the Oxides in the Solid State-Reaction in the Solid State at High Temperature. Z. Anorg. Allgem. Chem. 235:89-96
Adelskold V. (1938) X-ray Studies on Magneto-plumbite, PbO · 6Fe2O3, and Other Substances Resembling ‘Beta-Alumina’, Na2O · 11Al2O3. Alk. Kemi. Mineral Geol. 12A(29):1-9
Toropov N.A. (1939) Crystalloptical Analysis of Strontium Aluminates. Compt. Rend. Acad. Sci. U.R.S.S. 23:74-75
Toropov N.A., Stukalova M.M. (1940) Replacement of Sodium in Crystals of “\({\upbeta}\)-alumina” With Calcium, Strontium and Barium. Compt. Rend. Acad. Sci. U.R.S.S. 27:974-977
P.S. Dear, X-ray Diffraction Data for Silicates, Aluminates and Aluminosilicates of Strontium, Bull. Virginia Ploytechn. Inst., 50(6), Eng. Expt. Stat., 1957, 117, p 1-15
F. Massazza, The System SrO-Al2O3. Chim. Ind. (Milan), 1959, 41, p 108-115 (in Italian); E. M. Levin, C. R. Robbins, and H. F. McMurdie, Ed., Phase Diagrams for Ceramists, American Ceramic Society, Colombus, OH, 1964, Fig. 294-295
Massazza F., Cannas M. (1959) Research on the System CaO-Al2O3-SrO. Ann. Chimica (Rome) 49:1342-1351, in Italian
Brisi C., Abbattista F. (1960) Searches on the Heats of Formation of the Strontium Aluminated One. Ann. Chimica 50:165-169, in Italian
Appendino P. (1972) Balances in a solid equilibria in the system SrO-BaO-Al2O3. Rev. Int. Hautes Temper. Refract. 9:297-302, in French
F. Ganits, T. Yu. Chemekova, and Yu. P. Udalov, The System SrO-Al2O3, Zh. Neorg. Khim., 24(2), p 471-475 (in Russian); R.S. Roth, T. Negas, and L.P. Cook, Ed., Phase Diagrams for Ceramists, American Ceramic Society, Colombus, OH, 1987, Fig. 6427
Song Y.K., Choi S.K., Moon H.S., Kim T.W., Mho S.I., Park H.L. (1997) Phase Studies of SrO-Al2O3 by Emission Signatures of Eu2+ and Eu3+. Mater. Res. Bull. 32(3):337-341
T. N. Nadezhina, E. A. Pobedimskaya, and N. V. Belov, Coordination Polyhedra of Strontium in the Structures Sodium strontium germanate Na4SrGe3O3[GeO4]3, Strontium germanate Sr3[Ge3O9], Strontium aluminate Sr4Al4O2[Al10O23] and bis(hydroxystrontium) tetrahydroxycuprate(II) Sr2(OH)2[Cu(OH)4]. Kristallografiya, 1980, 25, p 938-943 (Sov. Phys. Crystallogr., 1980, 25, p 537-540)
Wang M., Wang D., Lu G. (1998) Research on Fluorescence Spectra and Structure of Single-phase 4SrO · 7Al2O3:Eu2+ Phosphor Prepared by Solid-state Reaction Method. Mater. Sci. Engin. B 57:18-23
Lin Y., Tang Z., Zhang Z. (2001) Preparation of Long-afterglow Sr4Al14O25-based Luminescent Material and Its Optical Properties. Mater. Lett. 51:14-18
M. Capron, Synthesis and Characterization of Aluminates and Aluminosilicates of the Ambient Temperature to the Liquid at High Temperatures. Thesis, University of Orleans, 2001 (in French)
Andre D., Capron M. (2003) Crystallisation of Spray-dried Amorphous Precursors in the SrO-Al2O3 System: A DSC Study. J. Euro. Ceram. Soc. 23:2075-2081
Yamaguchi O., Narai A., Shimizu K. (1986) New Compound in the System SrO-Al2O3. J. Am. Ceram. Soc. 69:C36-C37
Kahlenberg V. (2002) Synthesis and Crystal Structure of Sr10Al6O19: A Derivative of the Perovskite Structure Type in the System SrO-Al2O3. Mat. Res. Bull. 37:715-726
Knacke O., Kubaschewski O., Hesselmann K. (1991) Thermochemical Properties of Inorganic Substances, 2nd ed. Springer-Verlag, Berlin, Heidelberg, New York, London
X. Zhang, Solid-State Combinatorial Chemistry Method and Its Application in Inorganic Luminescent Materials, Dissertation for post-doctor, Peking University, 2005 (in Chinese)
Risold D., Hallstedt B., Gauckler L.J. (1996) The Strontium-Oxygen System. Calphad 20(3):353-361
The SGTE Substance Database, Version 1994, SGTE (Scientific Group Thermodata Europe), Grenoble, France, 1994
Capron M., Florian P., Fayon F., Trumeau D., Hennet L., Gaihlanou M., Thiaudiere D., Landron C., Douy A., Massiot D. (2001) Local Structure and Dynamics of High Temperature SrO-Al2O3 Liquids Studied by 27Al NMR and Sr K-edge XAS Spectroscopy. J. Non-crystal. Solids 293-295:496-501
Hillert M., Jansson B., Sundman B., Agren J. (1985) A Two-sublattice Model for Molten Solutions with Different Tendency for Ionization. Metall. Trans. A 16A:261-266
Hallstedt B. (1992) Thermodynamic Assessment of the System MgO-Al2O3. J. Am. Ceram. Soc. 75(6):1497-1507
Hallstedt B. (1990) Assessment of the CaO-Al2O3 System. J. Am. Ceram. Soc. 73(1):15-23
Gutierrez G., Belonoshko A.B., Ahuja R., Johansson B. (2000) Structural Properties of Liquid Al2 O3: A Molecular Dynamics Study. Phys. Rev. E 61:2723-2729
G. Gruener, P. Odier, D. De Sousa Meneses, P. Florian, and P. Richet, Bulk and Local Dynamics in Glass-Forming Liquids: A Viscosity, Electrical Conductivity, and NMR study of Aluminosilicate melts, Phys. Rev. B, 2001, 64(2), Art.024206
Mao H., Hillert M., Selleby M., Sundman B. (2006) Thermodynamic Assessment of the CaO-Al2O3-SiO2 system. J. Am. Ceram. Soc. 89(1):298-308
Mao H., Fabrichnaya O., Selleby M., Sundman B. (2005) Thermodynamic Assessment of MgO-Al2O3-SiO2 System. J. Mater. Res. 20(4):975-986
Redlich O., Kister A. (1948) Algebraic Representation of Thermodynamic Properties and the Classification of Solutions. Ind. Eng. Chem. 40:345-348
Andersson J.-O., Helander T., Hoglund L., Shi P., Sundman B. (2002) Thermo-Calc & DICTRA Computational Tools for Materials Science. Calphad 26(2):273-312
Acknowledgments
The authors thank the National Natural Science Foundation of China for financial support No. 50204002, and Dr. Bo Sundman for the Thermo-Calc program package.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ye, X., Zhuang, W., Wang, J. et al. Thermodynamic Description of \({\hbox{SrO-Al}_{2}\hbox{O}_{3}}\) System and Comparison with Similar Systems. J Phs Eqil and Diff 28, 362–368 (2007). https://doi.org/10.1007/s11669-007-9086-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-007-9086-x