Abstract
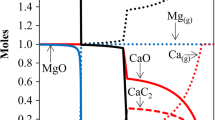
This work involves the production of magnesium in the form of Mg(OH)2 from serpentinite rock (nickel mine tailing) material followed by conversion into MgCO3 using a pressurised fluidised bed (PFB) reactor operating at 400°C–600°C and pressures up to 2.85 MPa. Our approach is rooted in the thermodynamic fact that the reaction between Mg(OH)2 and gaseous CO2 forming MgCO3 and water releases significant amounts of heat. The main problem is, however, the chemical kinetics; the reaction is slow and has to be accelerated in order to be used in an economically viable process for large-scale (∼1 Mt/a) CO2 sequestration. We have constructed a labscale PFB reactor test-setup for optimising the carbonation reaction. At high enough temperatures and conversion levels the reaction should provide the heat for the proceeding Mg(OH)2 production step, making the overall process energy neutral. So far we have been able to achieve a conversion degree of 26% at 500°C and 2.85 MPa after 30 min (particle size 125–212 μm). In this paper the test facility and our latest results and progress on CO2 mineral carbonation are summarised. Also, the possible integration of the iron as a feedstock for iron and steel production will be briefly addressed. An interesting side-effect of this carbon dioxide capture and storage (CCS) route is that significant amounts of iron are obtained from the serpentinite rock material. This is released during the Mg(OH)2 production and can be of great interest to the iron- and steel producing sector, which at the same time is Finland’s largest CO2 producer.
Similar content being viewed by others
References
Seifritz W. CO2 disposal by means of silicates. Nature, 1990, 345: 486
IPCC. IPCC Special Report on Carbon Dioxide Capture and Storage. Cambridge, United Kingdom: Cambridge University Press, 2005, 1–431
Eloneva S, Teir S, Salminen J, Fogelholm C, Zevenhoven R. Fixation of CO2 by carbonating calcium derived from blast furnace slag. Energy, 2008, 33: 1461–1467
Morhart A. New thermal storage tanks. Sun & Wind Energy, 2009, 2: 62–65
Hayes C Q. US Patent, WO 99/11455, 1999-09-04
Nduagu E I. Mineral carbonation: preparation of magnesium hydroxide [Mg(OH)2] from serpentinite rock. Dissertation for the Master Degree. Turku, Finland: Åbo Akademi Univ, 2008, 1–91
Zevenhoven R, Teir S, Eloneva S. Heat optimisation of a staged gas-solid mineral carbonation process for long-term CO2 storage. Energy, 2008, 33: 362–370
Robie R A, Hemingway B S, Fischer J R. Thermodynamic properties of minerals and related substances at 298.15 K and 1 bar (105 Pascals) pressure and at higher temperatures. US Geol Bull, 1979, 306: 1452
Kunii D, Levenspiel O. Fluidization Engineering. USA: Butterworth-Heinemann, 1991
Bearat H, McKelvy M J, Chizmeshya A V G, Gormley D, Nunez R, Carpenter R W, Squires K, Wolf G H. Carbon sequestration via aqueous olivine mineral carbonation: role of passivating layer formation. Environ Sci Technol, 2006, 40: 4802–4808
Shimizu T, Peglow M, Sakuno S, Misawa N, Suzuki N, Ueda H, Sasatsu H, Gotou H. Effect of attrition on SO2 capture by limestone under pressurized fluidized bed combustion conditions—comparison between a mathematical model of SO2 capture by single limestone particle under attrition condition and SO2 capture in a large-scale PFBC. Chemical Engineering Science, 2001, 56: 6719–6728
Shimizu T, Peglow M, Yamagiwa K, Tanaka M. Comparison among attrition-reaction models of SO2 capture by uncalcined limestone under pressurized fluidized bed combustion conditions. Chemical Engineering Science, 2003, 58: 3053–3057
Chen Z, Jim Lim C, Grace J R. Study of limestone particle impact attrition. Chemical Engineering Science, 2007, 62: 867–877
Lin C L, Wey M Y. Influence of hydrodynamic parameters on particle attrition during fluidization at high temperature. Korean J Chem Eng, 2005, 22: 154–160
Zhang Z, Ghadiri M. Impact attrition of particulate solids. Part 2: Experimental work. Chemical Engineering Science, 2002, 57: 3671–3686
Scala F, Cammarota A, Chirone R, Salatino P. Comminution of limestone during batch fluidized-bed calcination and sulfation. AIChE Journal, 1997, 43: 363–373
Scala F, Salatino P. Limestone fragmentation and attrition during fluidized bed oxyfiring. Fuel, 2009, doi: 10.1016/j.fuei.2009.03.024
Nikulshina V, Gálvez M E, Steinfeld A. Kinetic analysis of the carbonation reactions for the capture of CO2 from air via the Ca(OH)2-CaCO3-CaO solar thermochemical cycle. Chem Eng J, 2007, 129: 75–83
Ross S M. Peirce’s criterion for the elimination of suspect experimental data. J Eng Technol, 2003, Fall-edition: 1–12
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fagerlund, J., Nduagu, E., Romão, I. et al. A stepwise process for carbon dioxide sequestration using magnesium silicates. Front. Chem. Eng. China 4, 133–141 (2010). https://doi.org/10.1007/s11705-009-0259-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11705-009-0259-5