Abstract
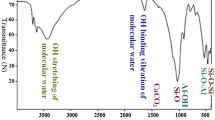
Equilibrium, kinetic and thermodynamic aspects of the adsorption of copper ions from an aqueous solution using linear alkylbenzene sulfonate (LABORATORIES) modified bentonite (organo-bentonite) are reported. Modification of bentonite was performed via microwave heating with a concentration of LABORATORIES surfactant equivalent to 1.5 times that of the cation exchange capacity (CEC) of the raw bentonite. Experimental parameters affecting the adsorption process such as pH, contact time and temperature were studied. Several adsorption equations (e.g., Langmuir, Freundlich, Sips and Toth) with temperature dependency were used to correlate the equilibrium data. These models were evaluated based on the theoretical justifications of each isotherm parameter. The Sips model had the best fit for the adsorption of copper ions onto organo-bentonite. For the kinetic data, the pseudo-second order model was superior to the pseudo-first order model. Thermodynamically, the adsorption of copper ions occurs via chemisorption and the process is endothermic (ΔH 0>0), irreversible (ΔS 0>0) and nonspontaneous (ΔG 0>0).
Similar content being viewed by others
References
Huang C C, Su Y J. Removal of copper ions from wastewater by adsorption/electrosorption on modified activated carbon cloths. Journal of Hazardous Materials, 2010, 175(1–3): 477–483
Zhao G, Zhang H, Fan Q, Ren X, Li J, Chen Y, Wang X. Sorption of copper(II) onto super-adsorbent of bentonite-polyacrylamide composites. Journal of Hazardous Materials, 2010, 173(1–3): 661–668
Fu F, Wang Q. Removal of heavy metal ions from wastewaters: a review. Journal of Environmental Management, 2011, 92(3): 407–418
Gök O, Ozcan A, Erdem B, Ozcan A S. Prediction of the kinetics, equilibrium and thermodynamic parameters of adsorption of copper (II) ions onto 8-hydroxyquinoline immobilized bentonite. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2008, 317(1–3): 174–185
Liu Y, Cao Q, Luo F, Chen J. Biosorption of Cd2+, Cu2+, Ni2+ and Zn2+ ions from aqueous solutions by pretreated biomass of brown algae. Journal of Hazardous Materials, 2009, 163(2–3): 931–938
Chen Z, Ma W, Han M. Biosorption of nickel and copper onto treated alga (Undaria pinnatifida): application of isotherm and kinetic models. Journal of Hazardous Materials, 2008, 155(1–2): 327–333
Anirudhan T S, Radhakrishnan P G. Thermodynamics and kinetics of adsorption of Cu(II) from aqueous solutions onto a new cation exchanger derived from tamarind fruit shell. Journal of Chemical Thermodynamics, 2008, 40(4): 702–709
Nathaniel E, Kurniawan A, Soetaredjo F E, Ismadji S. Organobentonite for the adsorption of Pb(II) from aqueous solution: temperature dependent parameters of several adsorption equations. Desalination and Water Treatment 2011, 36: 1–9
Kurniawan A, Sisnandy V O A, Trilestari K, Sunarso J, Indraswati N, Ismadji S. Performance of durian shell waste as high capacity biosorbent for Cr(VI) removal from synthetic wastewater. Ecological Engineering, 2011, 37(6): 940–947
Monier M, Ayad DM, Wei Y, Sarhan A A. Adsorption of Cu(II), Co (II), and Ni(II) ions by modified magnetic chitosan chelating resin. Journal of Hazardous Materials, 2010, 177(1–3): 962–970
Acharya J, Sahu J N, Mohanty C R, Meikap B C. Removal of lead (II) from wastewater by activated carbon developed from Tamarind wood by zinc chloride activation. Chemical Engineering Journal, 2009, 149(1–3): 249–262
Erdem E, Karapinar N, Donat R. The removal of heavy metal cations by natural zeolites. Journal of Colloid and Interface Science, 2004, 280(2): 309–314
Yesi, Sisnandy F P, Ju Y H, Soetaredjo F E, Ismadji S. Adsorption of acid blue 129 from aqueous solutions onto raw and surfactantmodified bentonite. Adsorption Science and Technology, 2010, 28: 847–868
Rahardjo A K, Susanto M J J, Kurniawan A, Indraswati N, Ismadji S. Modified Ponorogo bentonite for the removal of ampicillin from wastewater. Journal of Hazardous Materials, 2011, 190(1–3): 1001–1008
Do D D. Adsorption Analysis: equilibria and kinetics. London: Imperial College Press, 1998
Ismadji S, Bhatia S K. A modified pore-filling isotherm for liquidphase adsorption in activated carbon. Langmuir, 2001, 17(5): 1488–1498
Bhattacharyya K G, Gupta S S. Kaolinite, montmorillonite, and their modified derivatives as adsorbents for removal of Cu(II) from aqueous solution. Separation and Purification Technology, 2006, 50(3): 388–397
Lin S H, Juang R S. Heavy metal removal from water by sorption using surfactant-modified montmorillonite. Journal of Hazardous Materials, 2002, 92(3): 315–326
Álvarez-Ayuso E, Garcia-Sanchez A. Removal of heavy metals from waste waters by natural and Na-exchanged bentonites. Clays and Clay Minerals, 2003, 51(5): 475–480
Karapinar N, Donat R. Adsorption behaviour of Cu2+ and Cd2+ onto natural bentonite. Desalination, 2009, 249(1): 123–129
Ijagbemi C O, Baek M H, Kim D S. Montmorillonite surface properties and sorption characteristics for heavy metal removal from aqueous solutions. Journal of Hazardous Materials, 2009, 166(1): 538–546
Lagergren S. About the theory of so-called adsorption of soluble substances. Kungliga Svenska Vetenskapsakademiens Handlingar, 1898, 24: 1–39
Blanchard G, Maunaye M, Martin G. Removal of heavy metals from waters by means of natural zeolites. Water Research, 1984, 18(12): 1501–1507
Plazinski W, Rudzinski W, Plazinska A. Theoretical models of sorption kinetics including a surface reaction mechanism: a review. Advances in Colloid and Interface Science, 2009, 152(1–2): 2–13
Kul A R, Koyuncu H. Adsorption of Pb(II) ions from aqueous solution by native and activated bentonite: kinetic, equilibrium and thermodynamic study. Journal of Hazardous Materials, 2010, 179(1–3): 332–339
Schneider R M, Cavalin C F, Barros M A S D, Tavares C R G. Adsorption of chromium ions in activated carbon. Chemical Engineering Journal, 2007, 132(1–3): 355–362
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sandy, Maramis, V., Kurniawan, A. et al. Removal of copper ions from aqueous solution by adsorption using LABORATORIES-modified bentonite (organo-bentonite). Front. Chem. Sci. Eng. 6, 58–66 (2012). https://doi.org/10.1007/s11705-011-1160-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11705-011-1160-6