Abstract
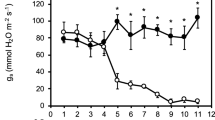
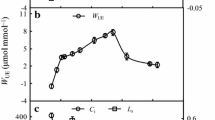
A field study was performed on triticale, field bean, maize and amaranth, to find differences between studied species in physiological alterations resulting from progressive response as injuries and/or acclimation to long-term soil drought during various stages of plant development. The measurements of leaf water potential, electrolyte leakage, chlorophyll a fluorescence, leaf gas exchange and yield analysis were done. A special emphasis was given to the measurements of the blue, green, red and far-red fluorescence. Beside, different ratios of the four fluorescence bands (red/far-red: F 690/F 740, blue/red: F 440/F 690, blue/far-red: F 440/F 740 and blue/green: F 440/F 520) were calculated. Based on both yield analysis and measurements of physiological processes it can be suggested that field bean and maize responded with better tolerance to the water deficit in soil due to the activation of photoprotective mechanism probably connected with synthesis of the phenolic compounds, which can play a role of photoprotectors in different stages of plant development. The photosynthetic apparatus of those two species scattered the excess of excitation energy more effectively, partially through its transfer to PS I. In this way, plants avoided irreversible and/or deep injuries to PS II. The observed changes in the red fluorescence emission and in the F v/F m for triticale and amaranth could have occurred due to serious and irreversible photoinhibitory injuries. Probably, field bean and maize acclimatized more effectively to soil drought through the development of effective mechanisms for utilising excitation energy in the photosynthetic conversion of light accompanied by the mechanism protecting the photosynthetic apparatus against the excess of this energy.
Similar content being viewed by others
Abbreviations
- C i :
-
Internal CO2 concentration
- D :
-
Drought stressed plants
- E :
-
Transpiration rate
- F v/F m :
-
Maximal quantum yield of PS II
- F v/F 0 :
-
Ratio of the variable fluorescence to the ground fluorescence
- FWC:
-
Field water capacity
- F 440 :
-
Fluorescence intensity at 440 nm
- F 520 :
-
Fluorescence intensity at 520 nm
- F 690 :
-
Fluorescence intensity at 690 nm
- F 740 :
-
Fluorescence intensity at 740 nm
- F 690/F 740 :
-
Ratio of the chlorophyll fluorescence intensity at 690 nm and 740 nm (red/far-red)
- F 440/F 690 :
-
Ratio of the fluorescence intensity at 440 nm and 690 nm (blue/red)
- F 440/F 740 :
-
Ratio of the fluorescence intensity at 440 nm and 740 nm (blue/far-red)
- F 440/F 520 :
-
Ratio of the fluorescence intensity at 440 nm and 520 nm (blue/green)
- G 1 and G 2 :
-
Generative stage of plant development
- g s :
-
Stomatal conductance
- IR:
-
Irrigated plants
- K :
-
Hydrothermal index
- LHC:
-
Light harvesting complex
- P N :
-
Net photosynthesis
- PS II:
-
Photosystem II
- PS I:
-
Photosystem I
- V:
-
Vegetative stage of plant development
- Ψw :
-
Leaf water potential
References
Agati G, Cerovic ZG, Moya I (2000) The effect of decreasing temperature up to chilling values on the in vivo F685/F735 chlorophyll fluorescence ratio in Phaseolus vulgaris an Pisum sativum: the role of the photosystem I contribution to the 735 nm fluorescence band. Photochem Photobiol 72:75–84
Baker NR, Rosenqvist E (2004) Applications of chlorophyll fluorescence can improve crop production strategies: an examination of future possibilities. J Exp Bot 55:1607–1621
Behera LM, Choudhury NK (1997) Changes in the chlorophyll fluorescence characteristics of chloroplasts from intact pumpkin cotyledons, caused by organ excision and kinetin treatment. Photosynthesis 34:161–168
Berkowitz GA, Chen C, Gibbs M (1983) Stromal acidification mediates in vivo water stress inhibition of nonstomatal-controlled photosynthesis. Plant Physiol 72:1123–1126
Bolhar-Nordenkampf HR, Long SP, Baker NR, Öquist G., Schreiber U (1989) Chlorophyll fluorescence as a probe of the photosynthetic competence of leaves in the field: a review of current instrumentation. Funct Ecol 3:497–514
Bolhar-Nordenkampf HR, Öquist G (1993) Chlorophyll fluorescence as a tool in photosynthesis research. In: Hall DO, Scurlock JMO, Bolhar-Nordenkampf HR, Leegood RC, Long SP (eds) Photosynthesis and production in a changing environment. A field and laboratory manual. Chapman & Hall, London
Buschmann C, Lichtenthaler HK (1998) Principles and characteristics of multi-colour fluorescence imaging of plants. J Plant Physiol 152:297–314
Buschmann C, Langsdorf G, Lichtenthaler HK (2000) Imaging of the blue, green, and red fluorescence emission of plants: an overview. Photosynthesis 38:483–491
Chaves MM, (1991) Effects of water deficits on carbon assimilation. J Exp Bot 42:1–16
Cornic G, Briantais JM (1991) Partitioning of photosynthetic electron flow between CO2 and O2 reduction in a C3 leaf (Phaceolus vulgaris L.) at different CO2 concentrations and during drought stress. Planta 183:178–184
Cornic G, Masacci A (1996) Leaf photosynthesis under drought stress. In: Baker NR (ed) Photosynthesis and the environment. Kluwer, Dordrecht, pp 347–366
Cornic G, Ghashghaie J, Genty B, Briantais JM (1992) Leaf photosynthesis is resistant to a mild drought stress. Photosynthesis 27:295–309
Dubey RS (1997) Photosynthesis in plants under stressful conditions. In: Mohammad P (ed) Handbook of photosynthesis. University of Arizona, Tuscon, Arizona. Marcel Dekker, Inc. New York
Edwards GE, Ku MSB (1987) Biochemistry of C3–C4 intermediates. In: Hatch MD, Boardman NK (eds) The biochemistry of plants, a comprehensive treatise. Photosynthesis, vol 10. Academic, New York
Flexas J, Escalona JM, Medrano H (1998) Down-regulation of photosynthesis by drought under field conditions in grapevine leaves. Aust J Plant Physiol 25:893–900
Flexas J, Bota J, Escalona JM, Sampol B, Medrano H (2002) Effects of drought on photosynthesis in grapevines under field conditions: an evaluation of stomatal and mesophyll limitations. Funct Plant Biol 29:461–471
Flexas J, Bota J, Galmés J, Medrano H, Ribas-Carbó M (2006) Keeping a positive carbon balance under adverse conditions: responses of photosynthesis and respiration to water stress. Physiol Plant 127:343–352
Havaux M (1992) Stress tolerance of photosystem II in vivo. Plant Physiol 100:424–432
Kitajima M, Butler WL (1975) Excitation spectra for photosystem I and photosystem II in chloroplasts and the spectral characteristics of the distribution of quanta between the two photosystems. Biochim Biophys Acta 408:297–305
Lang M, Lichtenthaler HK (1991) Changes in the blue-green and red fluorescence-emission spectra of beech leaves during the autumnal chlorophyll breakdown. J Plant Physiol 138:550–553
Lang M, Siffel P, Braunová Z, Lichtenthaler HK (1992) Investigations of the blue-green fluorescence emission of plant leaves. Bot Acta 105:435–440
Lang M, Lichtenthaler HK, Sowinska M, Summ P, Heisel F (1994) Blue, green and red fluorescence signatures and images of tabacco leaves. Bot Acta 107:230–236
Lawlor DW, Cornic G (2002) Photosynthetic carbon assimilation and associated metabolism in relation to water deficits in higher plants. Plant Cell Environ 25:275–294
Leegood RC, Edwards GE (1996) Carbon metabolism and photorespiration: temperature dependence in relation to other environmental factors. In: Baker NR (ed) Photosynthesis and the environment. Kluwer, Dordrecht, pp 191–221
Lichtenthaler HK (1996) Vegetation stress: an introduction to the stress concept in plants. J Plant Physiol 148:4–14
Lichtenthaler HK, Lang M, Sowinska M, Heisel F, Miehé JA (1996) Detection of vegetation stress via a new high resolution fluorescence imaging system. J Plant Physiol 148:599–612
Medrano H, Escalona JM, Bota J, Gulías J, Flexas J (2002) Regulation of photosynthesis of C3 plants in response to progressive drought: stomatal conductance as a reference parameter. Ann Bot 89:895–905
Mohammed GH, Binder WD, Gillies SL (1995) Chlorophyll fluorescence: a review of its practical forestry applications and instrumentation. Scand J For Res 10:383–410
Muller JE, Whitsitt MS (1996) Plant cellular response to water deficit. Plant Growth Regul 20:41–46
Noctor G, Veljovic-Jovanovic S, Driscoll S, Novitskaya L, Foyer CH (2002) Drought and oxidative load in the leaves of C3 plants: a predominant role for photorespiration? Ann Bot 89:841–850
Ort DR, Baker NR (2002) A photoprotective role for O2 as an alternative electron sink in photosynthesis? Curr Opin Plant Biol 5:193–198
van Rensburg L, Krüger GHJ (1993) Differential inhibition of photosynthesis (in vivo and in vitro), and changes in chlorophyll a fluorescence induction kinetics of four tobacco cultivars under drought stress. J Plant Physiol 141:357–365
Schweiger J, Lang M, Lichtenthaler HK (1996) Differences in fluorescence excitation spectra of leaves between stressed and non-stressed plants. J Plant Physiol 148:536–547
Šesták Z, Šiffel P (1997) Leaf-age related differences in chlorophyll fluorescence. Photosynthesis 33:347–369
Stober F, Lichtenthaler HK (1992) Changes of the laser-induced blue, green and red fluorescence signatures during greening of etiolated leaves of wheat. J Plant Physiol 140:673–680
Swain NK, Raval MK, Choudhury NK, Biswal UC (1990) Differential changes in fluorescence characteristics of photosystem 2 rich grana fraction during ageing in light and dark. Photosynthesis 24:135–142
Takács Z, Lichtenthaler HK, Tuba Z (2000) Fluorescence emission spectra of desiccation-tolerant cryptogamic plants during a rehydration-desiccation cycle. J Plant Physiol 156:375–379
Tambussi EA, Bartoli CG, Beltrano J, Guiamet JJ, Araus JL (2000) Oxidative damage to thylakoid proteins in water-stressed leaves of wheat (Triticum aestivum). Physiol Plant 108:398–400
Williams MH, Rosenqvist E, Buchhave M (1999) Response of potted miniature roses (Rosaxhybrida) to reduced water availability during production. J Hortic Sci Biotechnol 74:301–308
Yordanov I, Tsonev T, Goltsev V, Kruleva L, Velikova V (1997) Interactive effect of water deficit and high temperature on photosynthesis of sunflower and maize plants. 1. Changes in parameters of chlorophyll fluorescence induction kinetics and fluorescence quenching. Photosynthesis 33:391–402
Yordanov I, Velikova V, Tsonev T (2000) Plant responses to drought, acclimation, and stress tolerance. Photosynthesis 38:171–186
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Z. Starck.
Rights and permissions
About this article
Cite this article
Hura, T., Hura, K., Grzesiak, M. et al. Effect of long-term drought stress on leaf gas exchange and fluorescence parameters in C3 and C4 plants. Acta Physiol Plant 29, 103–113 (2007). https://doi.org/10.1007/s11738-006-0013-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11738-006-0013-2