Abstract
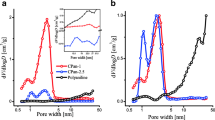
Taking the nano-sized carbon black and aniline monomer as precursor and (NH4)2S2O6 as oxidant, the well coated C/polyaniline(C/PANI) composite materials were prepared by in situ polymerization of the aniline on the surface of well-dispersed nano-sized carbon black for supercapacitor. The micro-structure of the C/PANI composite electrode materials were analyzed by SEM. The electrochemical properties of C/ PANI and PANI composite electrode were characterized by means of the galvanostatic charge-discharge experiment, cyclic voltammetric measurement and impedance spectroscopy analysis. The results show that by adding the nano-sized carbon black in the process of chemical polymerization of the aniline, the polyaniline can be in situ polymerized and well-coated onto the carbon black particles, which may effectively improve the aggregation of particles and the electrolyte penetration. What’s more, the maximum of specific capacitance of C/PANI electrode 437.6 F · g−1 can be attained. Compared with PANI electrode, C/PANI electrode shows more desired capacitance characteristics, smaller internal resistance and better cycle performance.
Similar content being viewed by others
References
LI Wen-cui, Reichenauer G. Carbon aerogel derived from cresol-resorcinol-formaldehyde for supercapacitor [J]. Carbon, 2002, 40(2): 2955–2959.
Pell W G, Conway B E. Analysis of non-uniform charge/discharge and rate effects in porous carbon capacitance containing sub-optimal electrolyte concentration[J]. Journal of Electroanalytical Chemistry, 2000, 491(1–2): 9–21.
Vix-Guterl C, Saadallah S, Jurewicz K, et al. super-capacitor electrodes from new ordered porous carbon materials obtained by a templating procedure[J]. Materials Science and Engineering, 2004, 108(2): 148–155.
WU Meng-qiang, Snook G A, George C, et al. Redox deposition of manganese oxide on graphite for supercapacitors[J]. Electrochemistry Communications, 2004, 6(5): 499–504.
Belanger D, REN Xiao-ming, LIU Ping, et al. Characterization and long-term performance of polyaniline-based electrochemical capacitors[J]. Journal of the Electrochemical Society, 2000, 147(3): 2923–2929.
Kim Y J, Horie Y, Matsuzawa Y, et al. Structure features necessary to obtain a high specific capacitance in electric double layer capacitor[J]. Carbon, 2004, 42(3): 2423–2432.
WANG Yong-gang, ZHANG Xiao-gang. Preparation and electrochemical capacitance of RuO2/TiO2 nanotubes composites[J]. Electrochemical Acta, 2004, 49(12): 1957–1962.
Gómez-Romero, Pedro, Chojak, et al. Hybrid organic-inorganic nanocomposite materials for application in solid-state electrochemical supercapacitors[J]. Electrochemistry Communications, 2003, 5 (2): 149–153.
Zbarsukov V, Khomenko V G, VChivikov S, et al. On the faradic and non-faradaic mechanisms of electrochemical processes in conducting polymers and some other reversible systems with solid-phase reagents[J]. Electrochimica Acta, 2001, 46(26): 4083–4094.
Conway B E. Transition from “supercapacitor” to “battery” behavior in electrochemical energy storage [J]. Journal of Electrochemical Society, 1991, 6(1): 1439–1448.
Passerini V, Vidakovic T, Dekanski A, et al. The properties of carbon-supported hydrous ruthenium oxide obtained from RuOxHy sol[J]. Electrochimica Acta, 2003, 48(25–26): 3805–3813.
Khomenko V, Frackowiak E, Beguin F. Determination of the specific capacitance of conducting polymer/nanotubes composite electrodes using different cell configuration[J]. Electrochimica Acta, 2004, 50(12): 2499–2506.
ZHOU Ying-ke, HE Ben-lin, ZHOU Wen-jia, et al. Electrochemical capacitance of well-coated single-walled carbon nanotube with polyaniline composite [J]. Electrochimica Acta, 2004, 49(2): 257–262.
Ryu K S, Lee Y G, Kim K M, et al. Electrochemical capacitor with chemically polymerized conducting polymer based on activated carbon as hybrid electrodes [J]. Synthetic Maetals, 2005, 153(1–3): 89–92.
HU Chi-Chang, Li W Y, Lin J Y. The capacitive characteristics of supercapacitors consisting of activated carbon fabric-polyaniline composites in NaNO3 [J]. Journal of Power Source, 2004, 137(1): 152–157.
Portet C, Taberna P L, Simon P, et al. Modification of Al current collector surface by sol-gel deposit for carbon-carbon supercapacitor applications[J]. Electrochimica Acta, 2004, 49(6): 905–912.
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item: Project(2005CB623703) supported by the National Basic Research Program of China; project(5JJ30103) supported by the Natural Science Foundation of Hunan Province
Rights and permissions
About this article
Cite this article
Lai, Yq., Li, J., Li, J. et al. Preparation and electrochemical characterization of C/PANI composite electrode materials. J Cent. South Univ. Technol. 13, 353–359 (2006). https://doi.org/10.1007/s11771-006-0048-y
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s11771-006-0048-y