Abstracts
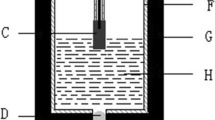
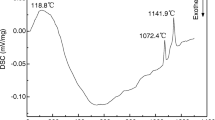
5Cu/(10NiO-NiFe2O4) cermet inert anodes were prepared by cold-pressing and sintering process, and the effect of superheat degree of melting K3AlF6-Na3AlF6-AlF3 on their anticorrosion performance was studied under electrolysis conditions. The results show that, the fluctuation of cell becomes small with increasing of superheat degree, which is helpful to inhibit the formation of cathodic encrustation; the concentration of impurities from inert anode in bath goes up to certain degree, but it is far smaller than those in traditional high-temperature bath. Increasing the superheat degree of melting K3AlF6-Na3AlF6-AlF3 has unconspicuous effect on the contents of impurities in cathodic aluminum. The total mass fractions of Fe, Ni and Cu in aluminum are 15.38% and 15.09% respectively under superheat degree of 95 and 195 °C. From micro-topography of anode used view, increasing the superheat degree can aggravate corrosion of metal Cu in inert anode, and has negative influence on electrical conductivity of electrode to some extent.
Similar content being viewed by others
References
GRJOTHEIM K, KROHN C, MALINOVSKY M, et al. Aluminium Electrolysis[M]. Dusseldorf: Aluminium-Verlag, 1982.
KENIRY J. The economics of inert anodes and wettable cathodes for aluminum reducation cells[J]. JOM, 2001, 53(5), 43–37.
de NORA V. Aluminium electrowinning—The future[J]. Aluminium, 2000, 76(12): 998–999.
KVANDEK H. Inert electrodes in aluminium electrolysis cells[C]// ECKERT C E. Light Metals 1999. California: TMS, 1999: 369–376.
HRYN J N, SADOWAY D R. Cell testing of metal anodes for the aluminium electrolysis[C]// DAS S K. Light Metals 1993. Colorado: TMS, 1993: 475–483.
YANG Jian-hong, LIU Ye-xiang, WANG Hua-zhang. The behaviour and improvement of SnO2-based inert anodes in aluminium electrolysis[C]// DAS S K. Light Metals 1993. Colorado: TMS, 1993: 493–495.
OLSEN E, ThONSTAD J. Nickel ferrite as inert anodes in aluminium electrolysis: part I. Material fabrication and preliminary testing[J]. Journal of Applied Electrochemistry, 1999, 29: 293–299.
LAI Yan-qing, TIAN Zhong-liang, QIN Qing-wei, et al. Solubility of complex oxide ceramic in Na3AlF6-Al2O3 melt[J]. Journal of Central South University: Nature Science, 2003, 34(3): 245–248. (in Chinese)
THONSTAD J, SOLHEIM A. The use of strongly acid low melting bath in aluminium electrolysis[J]. Aluminium, 1986, 62(12): 938–941.
SLEPPY W C, COCHRAN C N. Bench scale electrolysis of aluminium in sodium fluoride-aluminium fluride melts below 900 °C[C]// WARREN S, PETERSON. Light Metals 1979. Louisiana: TMS, 1979: 385–395.
GRJOTHEIM K, KVAND H. Physico-chemical properties of low-melting baths in aluminium electrolysis[J]. Metall, 1985, 39(6): 510–512.
BROWN C W. Laboratory experiments with low-temperature slurry-electrolyte alumina reduction cells[C]// PETERSON R D. Light Metals 2000. Tennessee: TMS, 2000: 391–395.
WANG Jia-wei, LAI Yan-qing, TIAN Zhong-liang, et al. Investigation of 5Cu-(10NiO-NiFe2O4) inert anode corrosion during low-temperature aluminum electrolysis[C]// SORLIE M. Light Metals 2007. Florida: TMS, 2007: 525–530.
LI Xi-zheng. Study on the sintering properties, electrical conductivity and corrosion resistance of XM/(10NiO-NiFe2O4) cermet[D]. Changsha: Central South University, 2006. (in Chinese)
TIAN Zhong-liang, LAI Yan-qing, ZHANG Gang, et al. Preparation of NiFe2O4-Cu based cermet inert anodes in aluminium electrolysis[J]. The Chinese Journal of Nonferrous Metals, 2003, 13(6): 1540–1545. (in Chinese)
ZHANG Gang, LAI Yan-qing, TIAN Zhong-liang, et al. Preparation of nickel ferrite based cermets for aluminum electrolysis[J]. Journal of Material Science and Engineering, 2003, 21(4): 44–47. (in Chinese)
PIETRZYK S, OBLAKOWSKI R. Investigation of the concentration of the inert anodes in the bath and metal during aluminum electrolysis[C]// ECKERT C E. Light Metals 1999. California: TMS, 1999: 407–411.
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item: Project (2005CB623703) supported by the Major State Basic Research and Development Program of China
Rights and permissions
About this article
Cite this article
Wang, Jw., Lai, Yq., Tian, Zl. et al. Effect of electrolysis superheat degree on anticorrosion performance of 5Cu/(10NiO-NiFe2O4) cermet inert anode. J. Cent. South Univ. Technol. 14, 768–772 (2007). https://doi.org/10.1007/s11771-007-0146-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11771-007-0146-5