Abstract
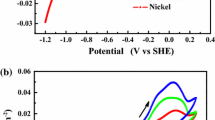
A study was carried out on kinetics of oxygen evolution on lead alloy anodes in sulphuric acidic electrolyte. The influence of alloy elements Ca, Ag and Sn on the overpotential of oxygen evolution was investigated. All anodes had been subjected to a pre-polarisation before the measurement of potential-current curves for oxygen evolution. The overpotential of oxygen evolution was found to be decreased when the alloy anode contained Ca and Ag, whereas it remained unchanged when the alloy anode contained Sn. For oxygen evolution on lead alloy anodes the TAFEL equation was valid. The b vulue for Pb and Pb-Ca anodes was approx. 100, for Pb-Ag, Pb-Ag-Ca anodes it was approx. 140. The a value for Pb-Ca, Pb-Ag, Pb-Ag-Ca anodes decreased with the increase of Ca or/and Ag content. The a and b value was not influenced by Sn in the anodes.
Similar content being viewed by others
References
Tainton U C, Taylor A G, Ehrlinger H P. Lead alloys for anodes in electrolytic production of zinc of high purity. Trans AIME, 1929, 85: 192
Hyvarinen O. The effect of silver and cobalt on the oxygen evolution at lead anodes: [dissertation]. Helsinki: The Helsinki University of Technology, 1972
Umetsu Y, Nozaka H, Tozawa K. Anodic behavior of Pb-Ag alloys in sulphuric acidic solution. J Min Metall Soc Jpn (in Japanese), 1985, 110: 375
Umetsu Y, Nozaka H, Tozawa K. Anodic behavior of Pb-Ag-Ca alloys in sulphuric acidic solution. J Min Mater Process Inst Jpn (in Japanese), 1989, 105: 249
Chen W. A study on kinetics of oxygen evolution on lead alloy anodes in sulphuric acidic electrolyte: [dissertation] (in German). Freiberg: Mining Academy of Freierg, 1993
Chen W, Hein K, Schmidt J. Oxygen evolution on lead dioxide anodes in sulphuric acidic electrolyte containing cobalt. Chemie-Ingenieur-Technik, 1993, 65: 587
Pourbaix M. Atlas of electrochemical equilibria in aqueous solution. Paris: Pergamon Press: 1966. 1
Mannheim R, Abdel-Reihim M, Reif W. Structure and properties of lead alloy anodes for the copper electrowinning. Metall (in German), 1986, 40:249
Author information
Authors and Affiliations
Additional information
Project supported by the Ministry of Economy of Germany
Synopsis of the first author Chen Wenmi, associate professor, born in May 1965, received Ph D degree at the Mining Academy of Freiberg in 1993, major research fields: electrode reaction kinetics, electroplating.
Rights and permissions
About this article
Cite this article
Chen, W., Guo, B. & Hein, K. Study on kinetics of oxygen evolution on lead alloy anodes. J Cent. South Univ. Technol. 4, 69–72 (1997). https://doi.org/10.1007/s11771-997-0035-y
Received:
Issue Date:
DOI: https://doi.org/10.1007/s11771-997-0035-y