Abstract
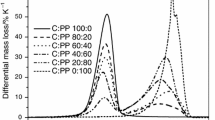
The pyrolysis of pure biomass, high density polyethylene (HDPE), polypropylene (PP) and polyethylene terephthalate (PET), plastic mixtures [HDPE+PP+PET (1: 1: 1)], and biomass/plastic mixture (9: 1, 3: 1, 1: 1, 1: 3 and 1: 9) were investigated by using a thermogravimetric analyzer under a heating rate at 10 °C/min from room temperature to 800 °C. Paper was selected as the biomass sample. Results obtained from this comprehensive investigation indicated that biomass was decomposed mainly in the temperature range of 290–420 °C, whereas thermal degradation temperature of plastic mixture is 390–550 °C. The percentage weight loss difference (W) between experimental and theoretical ones was calculated, which reached a significantly high value of (−)15 to (−)50% at around 450 °C in various blend materials. These thermogravimetric results indicate the presence of significant interaction and synergistic effect between biomass and plastic mixtures during their co-pyrolysis at the high temperature region. With increase in the amount of plastic mixture in blend material, the char production has diminished at final pyrolysis temperature range. Additionally, a kinetic analysis was performed to fit with TGA data, the entire pyrolysis processes being considered as one or two consecutive first order reactions.
Similar content being viewed by others
References
V. I. Sharypov, N. G. Beregovtsova, B. N. Kuznetsov, L. Membrado, V. L. Cebolla, N. Marin and J. V. Weber, J. Anal. Appl. Pyrolysis, 67, 325 (2003).
Y. Matsuzawa, M. Ayabe and J. Nishino, Polym. Deg. Stab., 71, 435 (2001).
L. Vivero, C. Barriocanal, R. Alvarez and M. A. Diez, J. Anal. Appl. Pyrolysis, 74, 327 (2005).
L. Zhou, Y. Wang, Q. Huang and J. Cai, Fuel Process. Tech., 87, 963 (2006).
N. Marin, S. Collura, V. I. Sharypov, N. G. Beregovtsova, S. V. Baryshnikov, B. N. Kutnetzov, V. Cebolla and J. V. Weber, J. Anal. Appl. Pyrolysis, 65, 41 (2002).
F. Shafizadeh, Adv. in Carbohydrate Chem., 23, 419 (1968).
A. Broido and M. Weenstein, In Proceedings of the 3rd International Conference on Thermal Analysis. Basel: Birkhauser Verlag, 285 (1971).
H. Higgins, J. Polym. Sci., 28, 645 (1958).
A. G. W. Bradbury, Y. Sakai and F. Shafizadeh, J. Appl. Polym. Sci., 23, 3271 (1979).
F. J. Kilzer and A. Broido, Pyrodynamics, 2, 151 (1965).
Jr. M. J. Antal, Advances in solar energy. New York: Solar Energy Society, pp. 61–111 (1983).
G. R. Ponder, G. N. Richards and T. T. Stevenson, J. Anal. Appl. Pyrolysis, 22, 217 (1992).
B. K. Kandola, A. R. Horrocks, D. Price and G. V. Coleman, J. Rev. Macromol. Chem. Phys., C36(4), 721 (1996).
S. L. Mardosky, editor. Thermal degradation of organic polymer, Interscience Publishers (1964).
M. Murata and T. Makino, Bull. Chem. Soc. Jpn., 12, 2414 (1973).
M. Murata and T. Makino, Bull. Chem. Soc. Jpn., 1, 192 (1975).
M. Dziêcio and J. Trzeszczyñski, J. Appl. Polym. Sci., 77, 1894 (2000).
B. Saha and A. K. Ghosal, Chem. Engg. J., 111, 39 (2005).
B. Saha, A. K. Maiti and A. K. Ghosal, Thermochimic. Acta, 444, 46 (2006).
M. Murata and T. Makino, Bull. Chem. Soc. Jpn., 7, 1241 (1975).
R. R. Stromberg, S. Straus and B. G. Achhammer, J. Polym. Sci., 35, 355 (1959).
R. Knuemann and H. Bockhorn, Combust. Sci. Tech., 101, 285 (1994).
C. H. Wu, C. Y. Chang, J. L. Hor, S. M. Shin and F. W. Chang, Canadian J. Chem. Eng., 72, 644 (1994).
A. Ballistreri, S. Foti, P. Maravigna, G. Montaudo and E. Scamporrino, Polymer, 22, 131 (1981).
C. A. Koufopanos, G. Maschio and A. Lucchesi, Canadian J. Chem. Eng., 67, 75 (1989).
T. Hatakeyama and F. X. Quinn, Thermal analysis — fundamentals and applications to polymer science, Wiley, Chichester (1999).
M. N. Nassar, Energy Sources, 21, 131 (1999).
K. G. Mansaray and A. E. Ghaly, Energy Sources, 21, 899 (1999).
J. A. Caballero, A. Marcilla and J. A. Conesa, J. Anal. Appl. Pyrolysis, 44, 75 (1997).
L. Helsen and E. vanden Buick, J. Anal. Appl. Pyrolysis, 56, 51 (2000).
E. Kastanaki, D. Vamvuka, P. Grammelis and E. Kakaras, Fuel Process. Technol., 77–78, 159 (2002).
V. I. Sharypov, N. Marin, N. G. Beregovtsova, S. V. Baryshnikov, B. N. Kuznetzov, V. L. Cebolla and J.V. Weber, J. Anal. Appl. Pyrolysis, 64, 15 (2002).
E. Jakab, G. Varhegyi and O. Faix, J. Anal. Appl. Pyrolysis, 56, 273 (2000).
F. J. Kilzer and A. Broido, Pyrodynamics, 2, 151 (1965).
I. Martin-Gullón, M. Esperanza and R. Font, J. Anal. Appl. Pyrolysis, 58–59, 635 (2001).
N. Horvat and F. T. T. Ng, Fuel, 78, 459 (1999).
H. Bockhorn, A. Hornung, U. Hornung and D. Schawaller, J. Anal. Appl. Pyrolysis, 48, 93 (1999).
A. W. Coats and J. F. Redfern, Nature, 68, 201 (1964).
V. K. Mustafa, O. Esber, K. Ozgen and H. Cahit, J. Anal. Appl. Pyrolysis, 45, 103 (1998).
P. R. Solomon, M. A. Serio, R. M. Carangelo and J. R. Markham, Fuel, 65, 82 (1986).
G. P. Ying, V. Enrique and P. Luis, Fuel, 75, 412 (1996).
M. J. Lazaro, R. Moliner and I. Suelves, J. Anal. Appl. Pyrolysis, 47, 111 (1998).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chattopadhyay, J., Kim, C., Kim, R. et al. Thermogravimetric characteristics and kinetic study of biomass co-pyrolysis with plastics. Korean J. Chem. Eng. 25, 1047–1053 (2008). https://doi.org/10.1007/s11814-008-0171-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-008-0171-6