Abstract
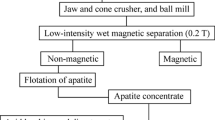
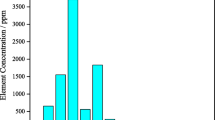
The extraction of rare earth elements from apatite concentrate of Chadormalu plant of Iran was studied with the dissolution of ore in nitric acid. The parameters of acidity: 60%, solid to liquid ratio: 30%, leaching time: 30 minute, agitation rate: 200 rpm, temperature: 60 °C and particle size (d80): 50 microns were determined as the optimum operational conditions. The recoveries of lanthanum, cerium, neodymium and yttrium were achieved at 74, 59, 72 and 73%, respectively, in the optimized conditions. Multivariable regression was used to predict La, Ce, Nd, Y and total REEs (Y+Nd+Ce+La) leaching recoveries, using experimental data from laboratory studies. It was achieved quite satisfactory correlations of 0.93, 0.98, 0.99, 0.97 and 0.99 for the prediction of Y, Nd, Ce, La and total REEs recoveries, respectively. It was shown that the proposed equations accurately reproduce the effects of operational variables on the different REEs recoveries, and can be used to optimize the REEs leaching plant.
Similar content being viewed by others
References
J. Ren, Sh. Song, A. Lopez-Valdivieso and Sh. Lu, Int. J. Miner. Process., 59, 237 (2000).
P. Martina, G. Carlota, A. Chevariera, C. Den-Auwerb and G. Panczerc, J. Nucl. Mater., 275, 268 (1999).
L. Hongfei, G. Fuqiang, Z. Zhifeng, L. Deqian and W. Zhonghuai, J.Alloys and Compounds, 408–412, 995 (2005).
P. Maestro and D. Huguenin, Alloys Compd., 255, 520 (1995).
J. Will, A. Mitterdorfer, C. Kleinlogel, D. Perednis and L. J. Gauckler, Solid State Ionics, 131, 79 (2000).
E. Jorjani, A. H. Bagherieh and B. Rezai, Jahad Daneshgahi, 26(4), 11 (2007).
National geosciences database of Iran, Chadormalu iron ore report, Brief information about metallic beneficiation plants, Iranian ministry of industry and mines (in Persian), 60–99 (2003).
F. Habashi, J. Chem. Technol. Biotechnol, GY, 35App, P. 5–14 (1985).
S. Naizhong, Z. Xiaowei, J. Qiong, Z. Weihong and L. Wuping, Korean J. Chem. Eng., 26, 1 (2009).
C. Koopman and G. J. Witkamp, Hydrometallurgy Journal, 58, 51 (2000).
V. P. Judin and H. E. Sund, Recovery of rare earths from secondary sources by solvent extraction, In: Hydrometallurgy ′81, Society of Chemical Industry, London, P. F4/1–F4/14 (1981).
J. I. Skorovarov, V. D. Kosynkin, S.D. Moiseev and N. N. Rura, J. Alloys and Compounds, 180, 71 (1992).
V. D. Kosynkin, S. D. Moiseev, C. H. Peterson and B. V. Nikipelov, J. Alloys and Compounds, 192, 118 (1993).
J. S. Preston, P. M. Cole, W. M. Craig and A. M. Feather, Hydrometallurgy, 41, 1 (1996).
C. Gupta, N. Krishnamurthy, Extractive metallurgy of rare earths, CRC press Inc., 1–540 (2005).
N. Lounamaa, T. Mattila, V. P. Judin and H. E. Sund, Recovery of rare earths phosphorus rock by solvent extraction. In: Proc. second Int. Congress Phosphorus Compounds, Institute Mondial du Phosphate, Paris, 759–768 (1980).
F. Habashi, a Textbook of Hydrometallurgy, Métallurgie Extractive Québec Enr., Quebec, Canada, P.1–689 (1999).
V. P. Judin and H. E. Sund, Recovery of rare earths from secondary sources by solvent extraction, In: Hydrometallurgy’ 81, Society of Chemical Industry, London, P. F4/1–F4/14 (1981).
J. I. Skorovarov, V. D. Kosynkin, S. D. Moiseev and N. N. Rura, J. Alloys and Compounds, 180, 71 (1992).
V. D. Kosynkin, S. D. Moiseev, C. H. Peterson and B. V. Nikipelov, J. Alloys and Compounds, 192, 118 (1993).
V. D. Kosynkin, A. K. Selivanovsky, V. M. Smolny, N.A. Tarasova and T. T. Fedulova, Incidental separation of rare earth concentrate in nitric acid and sulphuric acid processing of apatite fertilizer, IFA Technical sub-committee and committee meeting, 15–17 September 1999, Novgrod, Russia.
E. T. M. J. Martynowicz, Impurity uptake in calcium sulfate during phosphoric acid processing. PhD thesis, Delft University of Technology, The Netherlands (1994).
F. Habashi, Hydrometallurgy to solve phosphate processing, Industrial Minerals Magazine (2004).
E.V. Smirnova, I. N. Fedorova and I. Lozhkinb, Spectrochimica Acta Journal, 58(2), 329 (2003).
J. L. Chang, L. Gibaek, S. Won and E.Y. En, Korean J. Chem. Eng., 25, 568 (2008).
M. Ziabari, V. Mottaghitalab and A. Khodaparast Haghi, Korean J. Chem. Eng., 27, 340 (2010).
K. C. Young and K. Chul, Korean J. Chem. Eng., 10, 81 (1993).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jorjani, E., Bagherieh, A.H. & Chelgani, S.C. Rare earth elements leaching from Chadormalu apatite concentrate: Laboratory studies and regression predictions. Korean J. Chem. Eng. 28, 557–562 (2011). https://doi.org/10.1007/s11814-010-0383-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-010-0383-4