Abstract
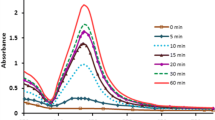
Colloidal silver nanoparticles were obtained by chemical reduction of silver nitrate in water and organic solvent with sodium borohydride. The effects of oxidant, reducing agent, stabilizer, and temperature, during the growth of silver nanoparticles were discussed. As the reaction proceeded in aqueous medium a characteristic plasmon absorption peak between 390-420 nm appeared as presence of silver nanoparticles. The peak intensities and shifting (blue or red) were altered in accordance with some applied factors. The formed silver nanoparticles were found to be with particles size range from 3 to 20 nm. The change rates of Ag+ ions to Ag0 in aqueous and organic solvent are strongly temperature dependent, although reduction can take place at room temperature. The silver nano-colloid with negative zeta potential also has been confirmed to be more stable. Obtained nanoparticles were characterized by UV-vis spectrophotometer, particle analyzer for zeta (ζ) potential, polydispersity index (PDI), and transmission electron microscope (TEM).
Similar content being viewed by others
References
C. Shing, V. Sharma, P. Naik, V. Khandelwal and H. Singh, Digest J. Nanomat Biostruct, 6(2), 535 (2011).
Y. Sun and Y. Xia, Science, 298, 2176 (2002).
X. Wu, H. Liu, J. Liu, K. N. Haley, J. A. Treadway, J. P. Larson, E. Ge, F. Peale and M. P. Bruchez, National Biotechnol., 21, 41 (2003).
A. Ruivo, C. Gomes, A. Lima, M. L. Botelho, R. Melo, A. Belchior and A. P. Matos, J. Cult. Herit, 9(Suppl. ), e134 (2008).
O. Bobin, M. Schvoerer, C. Ney, M. Rammah, B. Pannequin, E. C. Platamone, A. Daoulatli and R. P. Gayraud, Color Res. Appl., 28, 352 (2003).
N. Asare, C. Instanes, W. J. Sandberg, M. Refsnes, P. Schwarze, M. Kruszewski and G. Brunborg, Toxicology, 291(1-3), 65 (2011).
M. Ramos, D. A. Ferrer, R. R. Chianelli, V. Correa, J. M. Serrano and S. Flores, J. Nanomaterials, 1 (2011).
Y. Zheng, M. Xiao, S. Jiang, F. Ding and J. Wang, Nanoscale, 5, 788 (2013).
B. Tang, J. F. Wang, S. P. Xu, T. Afrin, J. L. Tao, W. Q. Xu, L. Sun and X. Wang, Chem. Eng. J., 185, 366 (2012).
B. Tang, J. Li, X. Hou, T. Afrin, L. Sun and X. Wang, Ind. Eng. Chem. Res., 52, 4556 (2013).
P. Li, J. Li, C. Wu, Q. Wu and J. Li, Nanotechnology, 16, 1912 (2005).
J. L. Elechiguerra, J. L. Burt, J. R. Morones, A. Camacho-Bragado, X. Gao, H. H. Lara and M. J. Yacaman, J. Nanobiotechnol., 3, 6 (2005), http://www.jnanobiotechnology. com/content/3/1/6.
R. Jin, Y. Cao, A. Mirkin, K. L. Kelly, G. C. Schatz and J. G. Zhang, Science, 294, 1901 (2001).
R. A. de Barros, C. R. Martins and W. M. de Azevedo, Synth. Met., 155, 35 (2005), DOI:10.1016/j.synthmet.2005.05.014.
K. L. Kelly, E. Coronado, L. L. Zhao and G. C. Schatz, J. Phys. Chem. B, 107, 668 (2002).
S. L. Kleinman, B. Sharma, M. G. Blaber, A.-I. Henry, N. Valley, R. G. Freeman, M. J. Natan, G. C. Schatz and R. P. V. Duyne, J. Am. Chem. Soc., 135, 301 (2013).
S. Szunerits and R. Boukherroub, Chem. Commun., 48, 8999 (2012).
A. I. Henry, J. M. Bingham, E. Ringe, L. D. Marks, G. C. Schatz and R. P. van Duyne, J. Phys. Chem. C, 115, 9291 (2011).
L. M. L. Marzan and I. Lado-Tourino, Langmuir, 12(15), 3585 (1996).
A. K. Rashid, R. K. Renat, G. Olga, E. Yuri and S. Thomas, Nanopart. Res., 11, 1193 (2009).
A. B. Smetana, K. J. Klabunde and C. M. Sorensen, J. Colloid Interface Sci., 284(2), 521 (2005).
K. J. Lee, B. H. Jun, J. Choi, Y. I. Lee, J. Joung and Y. S. Oh, Nanotechnology, 18, 335601 (5pp) (2007).
P. Y. Silvert, R. Herrera-Urbina, N. Duvauchelle and V. Vijayakrishnan, J. Mater. Chem., 6(4), 573 (1996).
Y. P. Sun, P. Atorngitjawat and M. J. Meziani, Langmuir, 17(19), 5707 (2001).
A. Henglein, Chem. Mater., 10(1), 444 (1998).
T. Kaushik, S. Mhatre and R. Parikh, Nanomed Nanotechnol. Bio. Med., 6(2), 257 (2010).
K. D. Kim, D. N. Han and H. T. Kim, Chem. Eng. J., 104, 55 (2004).
H. D. L. Van and C. F. Zukoski, Langmuir, 14, 7034 (1998).
J. P. Chen and L. L. Lim, Chemosphere, 49(4), 363 (2002).
A. Tao, P. Sinsermsuksaku and P. Yang, Angew. Chem. Int., 45, 4597 (2006).
H. D. L. Van and C. F. Zukoski, Langmuir, 17, 3128 (2001).
G. Wang, C. Shi, N. Zhao and X. Du, Mater. Lett., 61, 3795 (2007).
K. C. Song, S. M. Lee, T. S. Park and B. S. Lee, Korean J. Chem. Eng., 26(1), 153 (2009).
J. Liu, J. B. Lee, D. H. Kim and Y. Kim, Colloids Surf., A, 302, 276 (2007).
S. D. Solomon, M. Bahadory, A. V. Jeyarajasingam, S. A. Rutkowsky and C. Boritz, J. Chem. Ed., 84, 322 (2007).
J. Eastman and T. Cosgrove, Ed., p. 54, Blackwell UK (2005).
T. L. Farias., U. O. Koylu and M. G. J. Quant. Spectrosc. Radiar. Transfer., 55(3), 357 (1996).
W. R. Glomm, J. Dispersion Sci. Technol., 26, 389 (2005).
Victor Elias Torres Heredia, doctoral thesis (2011).
K. B. Mogensen and K. Kneipp, J. Phys. Chem. C, 118, 28075 (2014).
S. S. Khan, A. Mukherjee and N. Chandrasekaran, Water Res., 45, 5184 (2011).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Haque, M.N., Kwon, S. & Cho, D. Formation and stability study of silver nano-particles in aqueous and organic medium. Korean J. Chem. Eng. 34, 2072–2078 (2017). https://doi.org/10.1007/s11814-017-0096-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11814-017-0096-z