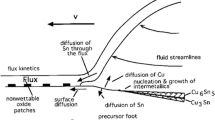
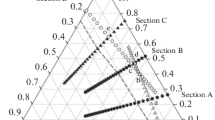
Abstract
Determining an accurate eutectic composition is more difficult than determining the corresponding eutectic temperature, a fact that was demonstrated in this study using a lead-free solder: the tin-rich Sn-Ag-Cu ternary eutectic. The solidification of this ternary eutectic involves the solid phases (Sn), Ag3Sn, and Cu6Sn5. The liquid is prone to supercooling, the intermetallics have steep liquidus surfaces (small phase fractions), and the coupled zone of eutectic microstructure formation is shifted toward silver-rich and copper-rich compositions. These issues were overcome by a combination of methods: preliminary thermodynamic calculation of the ternary phase diagram to anticipate difficulties, increased sensitivity of the thermal analysis, and a cycled heating and cooling method. The experimentally determined composition of the ternary eutectic is Sn-3.58±0.05Ag-0.96±0.04Cu at 217.2±0.2°C.
Similar content being viewed by others
References
W. Kurz and D. Fisher, Int. Met. Rev., 24 (1979), p. 177.
K.-W. Moon et al., J. Electron. Mater., 29 (2000), p. 1122.
E. Gebhardt and G. Petzow, Z. Metallkde., 50 (1959), p. 597.
V.N. Fedorov, O.E. Osinchev, and E.T. Yushkina, referenced in Phase Diagrams of Metallic Systems, Vol. 26, ed. N.V. Ageev and L.A. Petrova (Moscow, USSR: VINITI, 1982), pp. 149–150.
NCMS Report 0401 RE96, Lead-Free Solder Project (Ann Arbor, MI: National Center for Manufacturing Sciences, August 1997); CD-ROM, June 1998.
C.M. Miller, I.E. Anderson, and J.F. Smith, J. Electron. Mater., 23 (1994), p. 595.
M.E. Loomans and M.E. Fine, Metall. Mater. Trans., 31A (2000), p. 1155.
D.W. Henderson et al., J. Mater. Res., 17 (2002), p. 2775.
J.-Y. Park et al., J. Electron. Mater., 32 (2003), p. 1297.
Carol A. Handwerker, “NEMI Pb-free Solder Projects: Progress and Results” (Paper presented at IPC/JEDEC 4th International Conference on Lead-free Electronic Components and Assemblies, Frankfurt, Germany, 21–22 October 2003).
Tadatomo Suga, “Lead-free Roadmap 2002—Roadmap 2002 for Commercialization of Lead-free Soider, Official version 2.1,” Lead-free Soldering Roadmap Committee, JEITA (September 2002), http://tsc.jeita.or.jp/tsc/comms/7_easm/english/leadfree/index.htm.
Ursula R. Kattner, JOM, 53 (12) (2002), p. 45.
C.-S. Oh et al., J. Alloys Compds., 238 (1996), p. 155.
J.-H. Shim et al., Z. Metallkde., 87 (1996), p. 205.
H. Krieg, unpublished research, Max-Planck-Institut für Metallforschung, Stuttgart, Germany (1982).
Kil-Won Moon et al., “The Ternary Eutectic of Sn-Ag-Cu Solder Alloys,” Proceedings of SMTA 2000 International Conference (Edina, MN: Surface Mount Technology Assoc., 2000), p. 941.
R.I. Wu and J.H. Perepezko, Metall. Mater. Trans. A, 31A (2000), p. 497.
H. Biloni and W.J. Boettinger, Physical Metallurgy, 4th ed., ed. R.W. Cahn and P. Haasen (Dordrecht, The Netherlands: Elsevier Science, BV, 1996), p. 766.
Robert J. Schaefer and Daniel J. Lewis, “Directional Solidification in AgCuSn Eutectic Alloy,” submitted to Metall. Mater. Trans. A (2004).
Author information
Authors and Affiliations
Additional information
Author’s Note: Compositions in this paper are reported on a mass percent basis. The symbol (Sn) is used for the Sn phase to distinguish it from the element symbol Sn.
For more information, contact K.-W. Moon, National Institute of Science and Technology, Metallurgy Division, Materials Science and Engineering Laboratory, Gaithersburg, MD 20899, USA; (301) 975-6148; fax (301) 975-4553; e-mail kil-won.moon@nist.gov.
Rights and permissions
About this article
Cite this article
Moon, K.W., Boettinger, W.J. Accurately determining eutectic compositions: The Sn-Ag-Cu ternary eutectic. JOM 56, 22–27 (2004). https://doi.org/10.1007/s11837-004-0068-8
Issue Date:
DOI: https://doi.org/10.1007/s11837-004-0068-8