Abstract
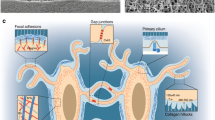
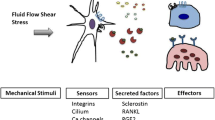
The osteocyte is the most abundant cell type of bone. There are approximately 10 times as many osteocytes as osteoblasts in adult human bone, and the number of osteoclasts is only a fraction of the number of osteoblasts. Our current knowledge of the role of osteocytes in bone metabolism is far behind our insight into the properties and functions of the osteoblasts and osteoclasts. However, the striking structural design of bone predicts an important role for osteocytes in determining bone structure. Over the past several years, the role of osteocytes as the professional mechanosensory cells of bone, and the lacunocanalicular porosity as the structure that mediates mechanosensing have become clear. Strain-derived flow of interstitial fluid through this porosity seems to mechanically activate the osteocytes, as well as ensure transport of cell signaling molecules, nutrients, and waste products. This concept explains local bone gain and loss—as well as remodeling in response to fatigue damage—as processes supervised by mechanosensitive osteocytes. Alignment during remodeling seems to occur as a result of the osteocyte’s sensing different canalicular flow patterns around the cutting cone and reversal zone during loading, therefore determining the bone’s structure.
Similar content being viewed by others
References and Recommended Reading
Kamioka H, Honjo T, Takano-Yamamoto T: A three-dimensional distribution of osteocyte processes revealed by the combination of confocal laser scanning microscopy and differential interference contrast microscopy. Bone 2001, 28:145–149.
Van der Plas A, Nijweide PJ: Isolation and purification of osteocytes. J Bone Miner Res 1992, 7:389–396.
Doty SB: Morphological evidence of gap junctions between bone cells. Calcif Tissue Int 1981, 33:509–512.
Cowin SC, Moss-Salentijn L, Moss ML: Candidates for the mechanosensory system in bone. J Biomed Eng 1991, 113:191–197.
Mullender MG, Huiskes R: Proposal for the regulatory mechanism of Wolff’s law. J Orthop Res 1995, 13:503–512.
Weinbaum S, Cowin SC, Zeng Y: A model for the excitation of osteocytes by mechanical loading-induced bone fluid shear stresses. J Biomech 1994, 27:339–360.
Klein-Nulend J, Van der Plas A, Semeins CM, et al.: Sensitivity of osteocytes to biomechanical stress in vitro. FASEB J 1995, 9:441–445.
Burger EH, Klein-Nulend J: Mechanotransduction in bone: role of the lacuno-canalicular network. FASEB J 1999, 13:S101-S112. This paper emphasizes the role of osteocytes as the mechanosensory cells of bone, and the lacunocanalicular structure that mediates mechanosensing. This concept explains local bone gain and loss— as well as remodeling in response to fatigue damage—as processes supervised by mechanosensitive osteocytes.
Burger EH, Klein-Nulend J, Smit TH: Strain-derived canalicular fluid flow regulates osteoclast activity in a remodeling osteon - a proposal. J Biomech 2003, In press. This paper proposes that bone alignment during remodeling occurs as a result of different canalicular flow patterns around the cutting cone and reversal zone during loading. It explains how the resorbing osteoclasts find their way through the pre-existing bone matrix.
Smit TH, Burger EH: Is BMU-coupling a strain-regulated phenomenon? A finite element analysis. J Bone Miner Res 2000, 15:301–307. This paper shows that the subsequent activation of osteoclasts and osteoblasts during remodeling of bone is a strain-related phenomenon.
Smit TH, Burger EH, Huyghe JM: A case for strain-induced fluid flow as a regulator of BMU-coupling and osteonal alignment. J Bone Miner Res 2002, 17:2021–2029. This paper demonstrates that cellular activity at a bone remodeling site is well related to local fluid patterns, which may explain the coordinated progression of a BMU.
Nijweide PJ, Mulder RJP: Identification of osteocytes in osteoblast-like cultures using a monoclonal antibody specifically directed against osteocytes. Histochemistry 1986, 84:343–350.
Van der Plas A, Aarden EM, Feyen JHM, et al.: Characteristics and properties of osteocytes in culture. J Bone Miner Res 1994, 9:1697–1704.
Tanaka-Kamioka K, Kamioka H, Ris H, Lim SS: Osteocyte shape is dependent on actin filaments and osteocyte processes are unique actin-rich projections. J Bone Miner Res 1998, 13:1555–1568.
Aarden EM, Wassenaar AM, Alblas MJ, Nijweide PJ: Immunocytochemical demonstration of extracellular matrix proteins in isolated osteocytes. Histochem Cell Biol 1996, 106:495–501.
Westbroek I, De Rooij KE, Nijweide PJ: Osteocyte-specific monoclonal antibody MAb OB7.3 is directed against Phex protein. J Bone Miner Res 2002, 17:845–853.
Skerry TM, Bitensky L, Chayen J, Lanyon LE: Early strainrelated changes in enzyme activity in osteocytes following bone loading in vivo. J Bone Miner Res 1989, 4:783–788.
El-Haj AJ, Minter SL, Rawlinson SCF, et al.: Cellular responses to mechanical loading in vitro. J Bone Miner Res 1990, 5:923–932.
Lean JM, Jagger CJ, Chambers TJ, Chow JW: Increased insulinlike growth factor I mRNA expression in rat osteocytes in response to mechanical stimulation. Am J Physiol 1995, 268:E318-E327.
Westbroek I, Ajubi NE, Alblas MJ, et al.: Differential stimulation of Prostaglandin G/H Synthase-2 in osteocytes and other osteogenic cells by pulsating fluid flow. Biochem Biophys Res Commun 2000, 268:414–419.
Westbroek I, Van der Plas A, de Rooij K, et al.: Expression of serotonin receptors in bone. J Biol Chem 2001, 276:28961–28968.
Klein-Nulend J, Semeins CM, Ajubi NE, et al.: Pulsating fluid flow increases nitric oxide (NO) synthesis by osteocytes but not periosteal fibroblasts-correlation with prostaglandin upregulation. Biochem Biophys Res Commun 1995, 217:640–648.
Piekarski K, Munro M: Transport mechanism operating between blood supply and osteocytes in long bones. Nature 1977, 269:80–82.
Dillaman RM: Movement of ferritin in the 2-day-old chick femur. Anat Rec 1984, 209:445–453.
Kufahl RH, Saha S: A theoretical model for stress-generated flow in the canaliculi-lacunae network in bone tissue. J Biomech 1990, 23:171–180.
Cowin SC, Weinbaum S, Zeng Y: A case for bone canaliculi as the anatomical site of strain generated potentials. J Biomech 1995, 28:1281–1296.
Wang N, Butler JP, Ingber DE: Mechanotransduction across the cell surface and through the cytoskeleton. Science 1993, 260:1124–1127.
Watson PA: Function follows form: generation of intracellular signals by cell deformation. FASEB J 1991, 5:2013–2019.
Binderman I, Shimshoni Z, Somjen D: Biochemical pathways involved in the translation of physical stimulus into biological message. Calcif Tissue Int 1984, 36:S82-S85.
Ajubi NE, Klein-Nulend J, Nijweide PJ, et al.: Pulsating fluid flow increases prostaglandin production by cultured chicken osteocytes - a cytoskeleton-dependent process. Biochem Biophys Res Commun 1996, 225:62–68.
Klein-Nulend J, Burger EH, Semeins CM, et al.: Pulsating fluid flow stimulates prostaglandin release and prostaglandin G/H synthase mRNA expression in primary mouse bone cells. J Bone Miner Res 1997, 12:45–51.
Klein-Nulend J, Sterck JGH, Semeins CM, et al.: Donor age and mechanosensitivity of human bone cells. Osteoporosis Int 2002, 13:137–146.
Rawlinson SCF, El-Haj AJ, Minter SLJ et al.: Loading-related increases in prostaglandin production in cores of adult canine cancellous bone in vitro: a role for prostacyclin in adaptive bone remodeling? J Bone Miner Res 1991, 6:45–1351.
Thompson DD, Rodan GA: Indomethacin inhibition of tenotomy-induced bone resorption. J Bone Miner Res 1988, 3:409–414.
Klein-Nulend J, Helfrich MH, Sterck JGH, et al.: EH: Nitric oxide response to shear stress by human bone cell cultures is endothelial nitric oxide synthase dependent. Biochem Biophys Res Commun 1998, 250:108–114.
Pitsillides AA, Rawlinson SCF, Suswillo RFL, et al.: Mechanical strain-induced NO production by bone cells - a possible role in adaptive bone (re)modeling. FASEB J 1995, 9:1614–1622.
Sterck JGH, Klein-Nulend J, Lips P, Burger EH: Response of normal and osteoporotic human bone cells to mechanical stress in vitro. Am J Physiol 1998, 274:E1113-E1120.
Rubin CT, Lanyon LE: Regulation of bone formation by applied dynamic loads. J Bone Joint Surg 1984, 66A:397–410.
Turner CH, Owan I, Takano Y, et al.: Nitric oxide plays a role in bone mechanotransduction. J Bone Miner Res 1995, 10(Suppl 1):235.
Zaman G, Pitsillides AA, Rawlinson SC, et al.: Mechanical strain stimulates nitric oxide production by rapid activation of endothelial nitric oxide synthase in osteocytes. J Bone Miner Res 1999, 14:1123–1131.
Busse R, Fleming I: Pulsatile stretch and shear stress: physical stimuli determining the production of endothelium derived relaxing factors. J Vasc Res 1998, 35:73–84.
Rossig L, Haendeler J, Hermann C, et al.: Nitric oxide down-regulates MKP-3 mRNA levels: involvement in endothelial cell protection from apoptosis. J Biol Chem 2000, 275:2552–2557.
Dimmeler S, Zeiher AM: Nitric oxide and apoptosis: another paradign for the double-edged role of nitric oxide. Nitric Oxide 1997, 1:275–281.
Bronckers ALJJ, Goei SW, Luo G, et al.: DNA fragmentation during bone formation in neonatal rodents assessed by transferasemediated en labeling. J Bone Miner Res 1996, 11:1281–1291.
Verborgt O, Gibson GJ, Schaffler MB: Loss of osteocyte integrity in association with microdamage and bone remodeling after fatigue in vivo. J Bone Miner Res 2000, 15:60–67.
Noble BS, Stevens H, Loveridge N, Reeve J: Identification of apoptotic changes in osteocytes in normal and pathological human bone. Bone 1997, 20:273–282.
Bronckers ALJJ, Goei W, Van Heerde WL, et al.: Phagocytosis of dying chondrocytes by osteoclasts in the mouse growth plate as demonstrated by annexin-V labeling. Cell Tissue Res 2000, 301:267–272.
MacIntyre I, Zaidi M, Alam ASMT, et al.: Osteoclast inhibition: an action of nitric oxide not mediated by cyclic GMP. Proc Natl Acad Sci U S A 1991, 88:2936–2940.
Huiskes R, Ruimerman R, van Lenthe GH, Janssen JD: Effects of mechanical forces on maintenance and adaptation from in trabecular bone. Nature 2000, 405:704–706.
Bakker AD, Soejima K, Klein-Nulend J, Burger EH: The production of nitric oxide and prostaglandin E2 by primary mouse bone cells is shear stress dependent. J Biomech 2001, 34:671–677.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Klein-Nulend, J., Nijweide, P.J. & Burger, E.H. Osteocyte and bone structure. Curr Osteoporos Rep 1, 5–10 (2003). https://doi.org/10.1007/s11914-003-0002-y
Issue Date:
DOI: https://doi.org/10.1007/s11914-003-0002-y